Prevalence and risk factors of long COVID 6–12 months after infection with the Omicron variant among nonhospitalized patients in Hong Kong
Jingyuan Luo, Jialing Zhang, Hiu To Tang, and Hoi Ki Wong are co-first authors.
Abstract
Long COVID has been reported among patients with COVID-19, but little is known about the prevalence and risk factors associated with long COVID 6–12 months after infection with the Omicron variant. This is a large-scale retrospective study. A total of 6242 out of 12 950 nonhospitalized subjects of all ages with SARS-CoV-2 infection (confirmed by polymerase chain reaction/rapid antigen test) during the Omicron dominant outbreak (December 31, 2021–May 6, 2022) in Hong Kong were included. Prevalence of long COVID, frequencies of symptoms, and risk factors were analyzed. Three thousand four hundred and thirty (55.0%) subjects reported at least one long COVID symptom. The most reported symptom was fatigue (1241, 36.2%). Female gender, middle age, obesity, comorbidities, vaccination after infection, having more symptoms, and presenting fatigue/chest tightness/headache/diarrhea in the acute stage of illness were identified as associated risk factors for long COVID. Patients who had received three or more doses of vaccine were not associated with a lower risk of long COVID (adjusted odds ratio 1.105, 95% confidence interval 0.985–1.239, p = 0.088). Among patients with at least three doses of vaccine, there was no significant difference in the risk of long COVID between the CoronaVac vaccine and BNT162b2 vaccine (p > 0.05). Omicron infection can lead to long COVID in a significant proportion of nonhospitalized patients 6–12 months after infection. Further investigation is needed to uncover the mechanisms underlying the development of long COVID and determine the impact of various risk factors such as vaccines.
1 INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has persisted since the end of 2019, 758 million cases and 6.8 million deaths were reported as of February 28, 2023.1 Apart from symptoms in the acute stage of infection, evidence has emerged that some individuals with COVID-19 experience persistent, relapsing, and remitting symptoms, leading to the introduction of the term “long COVID.”2, 3 The World Health Organization (WHO) defines the condition of COVID-19 patients lasting 3 months or more from the onset of the disease, with symptoms that persist for at least 2 months, and cannot be attributed to alternative diagnosis as “post COVID-19 syndrome” or “long COVID.”4 It may present in multiple symptoms in varied prevalence and severity, such as fatigue, cough, cognitive dysfunction, dyspnea, chest pain, and palpitations.2, 5-7 Given the scale of the pandemic, long COVID has brought a significant burden on healthcare systems globally, and its impact on the healthcare system warrants close monitor. Multiple symptoms of long COVID indicate this disorder could affect multiple organ systems with various possible pathogenic mechanisms. The hypotheses for its pathogenesis include persisting reservoirs of SARS-CoV-2 in tissues, immune dysregulation, microbiota dysbiosis, autoimmunity and primed immune cells from molecular mimicry, microvascular blood clotting with endothelial dysfunction, and dysfunctional neurological signaling.8, 9
Omicron variant of SARS-CoV-2 has rapidly become the dominant strain of the virus worldwide since late 2021.10 The Omicron dominated the fifth wave of the outbreak in Hong Kong has resulted in over 2.8 million confirmed cases and 13 120 death from December 2021 to January 2023.11 According to genome sequencing during the Hong Kong's fifth wave, most COVID-19 cases were caused by Omicron BA.2 (84.6%), only a small number of cases were caused by other sublineages, including Omicron BA.1 (11.5%) and Delta AY.127 (3.8%).12
Preliminary reports suggested that Omicron may cause less severe illness than Delta variant.13 Moreover, an observational study conducted in the United Kingdom found lower odds of having COVID symptoms over 4 weeks following Omicron infection compare to Delta in adult populations.14 Our previous retrospective study (n = 12 950) indicated that 17.1% of patients still sought medical consultation for the presence of symptoms over 4 weeks after the first positive test during the Omicron dominant outbreak.15 Other studies conducted in Eastern India (n = 524), the United States (n = 2197), the United Kingdom (n = 56003), and South Africa (n = 842) also found a significant number of individuals experiencing symptoms 4 weeks, 2 months, or 6 months after Omicron infection, with prevalence ranging from 4.5% to 18.5%.14, 16-18 However, currently there is a rare large-scale study investigating the prevalence of long-term (>6 months) consequences of Omicron infection.
Based on the studies of previous variants of concern including the wild type of SARS-CoV-2, Alpha and Beta, the potential risk factors associated with long COVID encompassed a broad range of variables, including demographic factors (e.g., gender and age), medical history (e.g., comorbidities), vaccination status, and symptoms during the acute phase of infection.2, 5-7, 19 In addition to these established risk factors, clinical factors that may influence the prevalence of long COVID, such as whether the patient was well treated at the acute stage of infection should also be considered. A comprehensive investigation of these diverse factors is crucial for identifying individuals at higher risk of developing long COVID and informing the development of effective prevention and treatment strategies.
This study aimed to investigate the prevalence and risk factors of long COVID among nonhospitalized COVID-19 survivors in Hong Kong who were infected with the Omicron variant.
2 METHODS
2.1 Study settings
This retrospective study was undertaken as part of the long COVID project at the Vincent V.C. Woo Chinese Medicine Clinical Research Institute, Hong Kong Baptist University (HKBU). We conducted a retrospective cohort study using electronic medical records and telephone follow-up visit records from HKBU. 12950 patients received consultation services with full electronic records from the University during the Omicron-dominated outbreak in Hong Kong. Chinese medicine physicians (CMPs) and research assistants (RAs) conducted a follow-up telephone visit between November 21, 2022 and January 20, 2023, to evaluate the health status of these patients 6–12 months after infection. The collected data included demographic, medical history, COVID-19 vaccination, treatment within 4 weeks of illness, and symptoms that occurred after the infection. All participants gave consent for data collection during the telephone visit.
2.2 Study population
We analyzed individuals who met the eligible criteria: (1) diagnosed with COVID-19 by polymerase chain reaction (PCR)/rapid antigen test (RAT) between December 31, 2021 and May 6, 2022 (fifth wave outbreak in Hong Kong); (2) nonhospitalized; (3) at least 6 months after diagnosis via HKBU teleconsultation system; (4) long COVID symptoms were not attributed to other newly developed diseases such as common cold or COVID-19 reinfection.
2.3 Outcomes
The primary outcome was the prevalence of long COVID. We applied the definition from WHO: COVID-19 patients lasting 3 months or more from the onset of the disease, with at least one symptom that persists for at least 2 months and cannot be attributed to another diagnosis.4 We identified 43 symptoms referring to WHO case definition, symptoms associated with SARS-CoV-2, and our previous study.4, 5, 15 Other symptoms could be input by CMPs/RAs in a text box. Secondary outcomes included: (1) impact of long COVID on normal life and work by self-reported in three levels: not at all, mild to moderate, and serious; (2) prevalence of individual long COVID symptoms. Specifically, as cough and fatigue were two key symptoms in our previous study, the self-reported severity level of these two symptoms were also collected on an 11-point scale (0–10 points, 0 is no symptom, 10 is the most serious as the patient can imagine); (3) Risk factors of long COVID. The covariates include gender, age, body mass index (BMI), days from first positive RAT/PCR test result to the first negative results (infection days), vaccine status (incomplete vaccination was defined to be less than three doses of vaccine; complete vaccination was at least three doses of vaccine20, 21), vaccination after the infection of SARS-CoV-2, treatment for acute stage of illness (within 4 weeks after infection22), patient's satisfaction of acute treatment, comorbidities, symptoms in the acute stage of infection (fever, chills, cough, sputum, dry throat, itching throat, sore throat, headache, chest tightness, abdominal distension, abdominal pain, nausea, diarrhea, myalgia and/or arthralgia, and fatigue).15 BMI was calculated from self-reported height and weight and classified according to criteria from the department of health in Hong Kong: underweight (<18.5 kg/m2), normal (18.5–<20 kg/m2), overweight (23.0–<25 kg/m2) and obese (≥25.0 kg/m2).23
The Government of the Hong Kong Special Administrative Region has provided the public with two COVID-19 vaccines: (1) the CoronaVac (Sinovac) vaccine, which uses the inactivated virus technology platform by Sinovac Biotech (Hong Kong) Limited, and (2) the BNT162b2 vaccine, which uses the messenger RNA (mRNA) technology platform by Fosun Pharma in collaboration with the German drug manufacturer BioNTech (BNT162b2 mRNA vaccine).24 Among patients who received at least three doses of vaccine, the types of vaccine (CoronaVac, BNT162b2, mixed two vaccines, other vaccines) were also be considered as a variable in the regression analysis of risk factors.
2.4 Statistical methods
Normally distributed continuous variables were reported as mean (SD) or 95% confidence interval (CI); nonnormally distributed continuous variables were described as median (interquartile range [IQR]). Categorical variables as frequencies and percentages. Logistic regression models were used to provide unadjusted and adjusted odds ratio (aOR) to assess the association between the risk factors for developing long COVID. Adjustments were made for age, gender, BMI, infection days, vaccination status, vaccination after infection, comorbidities, infection days, treatment for the acute stage of illness, and patient's satisfaction of treatment. The differences of continuous variables between cohorts with and without long COVID were assessed by Student's t test or nonparametric Mann–Whitney U test. As for categorical variables, the chi-squared test was used. As a sensitivity test, we analyzed the prevalence of symptoms among patients without any comorbidity. Statistical significance was defined as two-sided p value < 0.05. Missing values were not imputed. The statistical analyses were performed using the Statistical Packages of Social Sciences for Windows (SPSS; version 27.0).
2.5 Ethical approval
All patients provided informed consent at the start of the online consultation and follow-up visit in HKBU. The university exempted ethical approval for retrospective studies. All personal data were deidentified. The reporting of this study followed the recommendations of the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guideline.25 The checklist can be found in the Supporting Information File 1.
3 RESULTS
3.1 Participants
A total of 12 950 patients with confirmed SARS-CoV-2 during the Omicron-dominated outbreak in Hong Kong were included in the HKBU database, of whom 6814 (52.6%) had follow-up telephone survey records. After excluding 182 (2.7%) subjects who were diagnosed with a new disease after COVID, 341 (5.0%) were infected by SARS-CoV-2 again, 49 (0.7%) were hospitalized after the infection, 6242 nonhospitalized COVID-19 patients were included in the analysis. The study flowchart is presented in Figure 1.
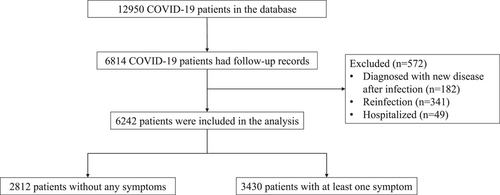
Table 1 summarizes the descriptive characteristics of the study population. The median time between the initial positive RAT/PCR test and the time of survey was 9 (IQR 8, 10) months. The median age of the study population was 47 (36, 60), with more female (4176, 66.9%). The median BMI of study population was 22.5 (20.3, 25.0), and 14.1% were obese (BMI ≥ 25.0 kg/m2). A total of 3590 (57.5%) patients had less than three doses of vaccination, and 1957 (31.4%) had an extra dose after infection of SARS-CoV-2. Among 2652 patients who received at least three doses of vaccine, 1187 (44.8%) only received the CoronaVac vaccine, and 1138 (42.9%) only received the BNT162b2 vaccine, 251 (9.5%) received both two vaccines, 19 (0.7%) received other types of vaccine outside of Hong Kong, and 57 (2.1%) did not provided vaccine information. There were 2020 (32.4%) participants who had at least one comorbidity before the diagnosis of COVID-19, the most prevalent comorbidity was hypertension (958, 15.3%). Most of the patients (5816, 93.4%) were symptomatic within the 4 weeks of diagnosis, and 5828 (93.4%) had treatments (e.g., nonsteroid anti-inflammation drugs, Chinese herbal medicine, antiviral drugs) in the acute stage of infection, and 4237 (67.9%) were satisfied with the treatment. The median infection duration was 7 (IQR 6, 9).
| Characteristic | Total (n = 6242) | Long COVID (n = 3430) | Recovered (n = 2812) | p Value |
|---|---|---|---|---|
| Gender | <0.001 | |||
| Female | 4176 (66.9) | 2483 (72.4) | 1693 (60.2) | |
| Male | 2066 (33.1) | 947 (27.6) | 1119 (39.8) | |
| Age | 47 (36, 60) | 48 (37, 60) | 45 (34, 60) | <0.001 |
| <18 | 476 (7.6) | 176 (5.1) | 300 (10.7) | <0.05 |
| 18–29 | 427 (6.8) | 196 (5.7) | 231 (8.2) | <0.05 |
| 30–39 | 1205 (19.3) | 657 (19.2) | 548 (19.5) | >0.05 |
| 40–49 | 1342 (21.5) | 792 (23.1) | 550 (19.6) | <0.05 |
| 50–59 | 1122 (18.0) | 677 (19.7) | 445 (15.8) | <0.05 |
| 60–69 | 1047 (16.8) | 589 (17.2) | 458 (16.3) | >0.05 |
| ≥70 | 623 (10.0) | 343 (10.0) | 280 (10.0) | >0.05 |
| BMI (kg/m2) | 22.5 (20.3, 25.0) | 22.5 (20.3, 25.4) | 22.3 (20.2–24.7) | 0.001 |
| Underweight (<18.5) | 311 (5.0) | 184 (5.4) | 127 (4.5) | >0.05 |
| Normal (18.5 < 23.0) | 1561 (25.0) | 901 (26.3) | 660 (23.5) | <0.05 |
| Overweight (23.0 < 25.0) | 621 (9.9) | 353 (10.3) | 268 (9.5) | >0.05 |
| Obese (≥25.0) | 882 (14.1) | 569 (16.6) | 313 (11.1) | <0.05 |
| Missing | 2867 (45.9) | 1423 (41.5) | 1444 (51.4) | <0.05 |
| Vaccine doses | 0.001 | |||
| <3 doses | 3590 (57.5) | 1908 (55.6) | 1682 (59.8) | |
| ≥3 doses | 2652 (42.5) | 1522 (44.4) | 1130 (40.2) | |
| Vaccination after infection | <0.001 | |||
| Yes | 1957 (31.4) | 1135 (33.1) | 882 (29.2) | <0.05 |
| No | 3373 (54.0) | 1848 (53.9) | 1525 (54.2) | >0.05 |
| Missing | 912 (14.6) | 447 (13.0) | 465 (16.5) | <0.05 |
| At least one comorbidity | 2020 (32.4) | 1226 (35.7) | 794 (28.2) | <0.001 |
| Hypertension | 958 (15.3) | 569 (16.6) | 389 (13.8) | 0.003 |
| Diabetes | 376 (6.0) | 206 (6.0) | 170 (6.0) | 0.948 |
| Hyperlipidemia | 407 (6.5) | 271 (7.9) | 136 (4.8) | <0.001 |
| Respiratory disease | 114 (1.8) | 84 (2.4) | 30 (1.1) | <0.001 |
| Cardio-cerebrovascular disease | 147 (2.4) | 100 (2.9) | 47 (1.8) | 0.001 |
| Liver disease | 44 (0.7) | 30 (0.9) | 14 (0.5) | 0.77 |
| Renal disease | 14 (0.2) | 9 (0.3) | 5 (0.2) | 0.482 |
| Psychological disease | 37 (0.6) | 23 (0.7) | 14 (0.5) | 0.377 |
| Neurological disease | 11 (0.2) | 8 (0.2) | 3 (0.1) | 0.364 |
| Digestive disease | 37 (0.6) | 30 (0.9) | 7 (0.2) | 0.001 |
| Endocrine disease | 78 (1.2) | 53 (1.5) | 25 (0.9) | 0.020 |
| Eyes, ear, nose, throat disease | 42 (0.7) | 37 (1.1) | 5 (0.2) | <0.001 |
| Other disease | 167 (2.7) | 114 (3.3) | 53 (1.9) | <0.001 |
| Impact of long COVID on normal life | ||||
| Not at all | 853 (24.9) | 853 (24.9) | 0 | N/A |
| Mild-moderate | 1781 (51.9) | 1781 (51.9) | 0 | N/A |
| Severe | 355 (10.3) | 355 (10.3) | 0 | N/A |
| Missing | 441 (12.9%) | 441 (12.9%) | 0 | N/A |
| At least one symptom within 4 weeks of diagnosis | 5816 (93.2) | 3221 (93.9) | 2595 (92.3) | 0.011 |
| Days between first positive and first negative | 7 (6, 9) | 7 (7, 10) | 7 (6, 9) | <0.001 |
| Treatment within 4 weeks of diagnosis | 5828 (93.4) | 3279 (95.6) | 2549 (90.6) | <0.001 |
| Satisfied with the treatment | <0.001 | |||
| Yes | 4237 (67.9) | 2299 (67.0) | 1938 (68.9) | >0.05 |
| No | 648 (10.4) | 439 (12.8) | 209 (7.4) | <0.05 |
| Missing | 1357 (21.7) | 692 (20.2) | 665 (23.6) | <0.05 |
| Follow-up time (months) | 9 (8, 10) | 9 (8, 10) | 9 (8, 9) | <0.001 |
- Abbreviations: BMI, body mass index; N/A, not applicable.
3.2 The prevalence of long COVID after 6-12 months of infection
There were 3430 (55.0%) patients reported at least one persistent long COVID symptom during the follow-up visit, and 2812 (45%) without any symptom. Compared with recovered patients (n = 2812), long COVID patients (n = 3430) were older (48, 37–60 vs. 45, 34–60, p < 0.001), in a higher percentage of female (2483, 72.4% vs. 1693, 60.2%, p < 0.001), obese (569, 16.6% vs. 313, 11.1%, p < 0.001), vaccinated at least three doses (1522, 44.4% vs. 1130, 40.2%, p < 0.001), and more patients with at least one comorbidity diagnosed before the infection (1226, 35.7% vs. 794, 28.2%, p < 0.001). In addition, long COVID patients reported longer infection days (7, 7–10 vs. 7, 6–9, p < 0.001). Also, fewer of them were satisfied with the treatment during the acute stage of diagnosis (439, 12.8% vs. 209, 7.4%, p < 0.001). In the long COVID cohort (n = 3430), around half (1781, 51.9%) of patients reported mild to moderate affection of their normal life, 355 (10.3%) reported being seriously affected, and 853 (24.9%) were not affected at all.
3.3 Symptoms
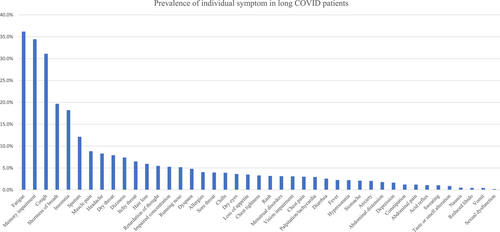
The prevalence of the 43 symptoms among long COVID patients are shown in Figure 2. There were six symptoms with a prevalence of more than 10%, which included fatigue (1241, 36.2%), hypomnesia (1181, 34.4%), cough (1067, 31.1%), shortness of breath (675, 19.7%), insomnia (625, 18.2%), and production of sputum (416, 12.1%). Nine symptoms with a prevalence of more than 5%, including muscle pain (303, 8.8%), headache (285, 8.3%), dry throat (272, 7.9%), dizziness (254, 7.4%), itchy throat (223, 6.5%), hair loss (204, 5.9%), retardation of though (188, 5.5%), impaired concentration (181, 5.3%), and running nose (178, 5.2%). The remaining 28 symptoms with a prevalence of less than 5%. The median severity level of fatigue and cough were 6 (IQR 5–7) and 5 (IQR 3–6), respectively. We analyzed the symptom profiles in patients without any comorbidity (n = 2204). The six symptoms with the highest prevalence in patients without any comorbidity were still fatigue (842, 38.2%), hypomnesia (787, 35.7%), cough (721, 32.7%), shortness of breath (432, 19.6%), insomnia (412, 18.7%), and production of sputum (254, 11.5%).
3.4 Risk factors for the development of long COVID
The association between risk factors and the development of long COVID is presented in Table 2. Six to twelve months after infection, there were several demographic and clinical risk factors significantly associated with the occurrence of long COVID. Compared with female, male was at a lower risk (aOR 0.590, 95% CI 0.525–0.663, p < 0.001). Compared with the 18–29 years age group, patients in 30–39 years (1.276, 1.004–1.620, p = 0.046), 40–49 years (1.495, 1.178–1.898, p = 0.001), 50–59 years (1.497, 1.168–1.918, p = 0.001) were more likely to report long COVID symptoms. Compared with patients with a normal range of BMI (18.5–23 kg/m2), obese patients were significantly associated with an increased risk of long COVID symptoms (1.321, 1.102–1.583, p = 0.003).
| Variables | Unadjusted OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Female | Ref. | Ref. | ||||
| Male | 0.577 | 0.519–0.642 | 0.000 | 0.590 | 0.525–0.663 | 0.000 |
| Age (18–29 as reference) | 0.000 | |||||
| <18 | 0.691 | 0.530–0.902 | 0.007 | 0.897 | 0.668–1.203 | 0.468 |
| 18–29 | Ref. | Ref. | ||||
| 30–39 | 1.413 | 1.132–1.763 | 0.002 | 1.276 | 1.004–1.620 | 0.046 |
| 40–49 | 1.697 | 1.363–2.113 | 0.000 | 1.495 | 1.178–1.898 | 0.001 |
| 50–59 | 1.793 | 1.432–2.245 | 0.000 | 1.497 | 1.168–1.918 | 0.001 |
| 60–69 | 1.516 | 1.209–1.900 | 0.000 | 1.243 | 0.960–1.609 | 0.099 |
| ≥70 | 1.444 | 1.127–1.849 | 0.004 | 1.013 | 0.753–1.362 | 0.933 |
| Vaccine doses ≥ 3 doses | 1.187 | 1.073–1.314 | 0.001 | 1.105 | 0.985–1.239 | 0.088 |
| Vaccination after infection | ||||||
| Yes | 1.139 | 1.018–1.275 | 0.023 | 1.470 | 1.199–1.804 | 0.000 |
| Missing | 0.793 | 0.685–0.918 | 0.002 | 1.659 | 1.339–2.056 | 0.000 |
| BMI | ||||||
| Underweight (<18.5) | 1.061 | 0.829–1.359 | 0.637 | 1.152 | 0.887–1.497 | 0.289 |
| Normal (18.5–<23.0) | Ref. | Ref. | ||||
| Overweight (23.0–<25.0) | 0.965 | 0.800–1.164 | 0.709 | 0.991 | 0.814–1.207 | 0.926 |
| Obese (≥25.0) | 1.332 | 1.123–1.579 | 0.001 | 1.321 | 1.102–1.583 | 0.003 |
| Missing | 0.722 | 0.638–0.817 | 0.000 | 0.743 | 0.649–0.852 | 0.000 |
| Comorbidity | ||||||
| Hypertension | 1.239 | 1.077–1.425 | 0.003 | 1.151 | 0.961–1.379 | 0.127 |
| Diabetes | 0.993 | 0.805–1.224 | 0.948 | 0.739 | 0.576–0.949 | 0.018 |
| Hyperlipidemia | 1.688 | 1.365–2.087 | 0.000 | 1.732 | 1.347–2.228 | 0.000 |
| Respiratory disease | 2.328 | 1.530–3.543 | 0.000 | 2.050 | 1.324–3.172 | 0.001 |
| Cardio-cerebrovascular disease | 1.767 | 1.245–2.508 | 0.001 | 1.666 | 1.145–2.423 | 0.008 |
| Liver disease | 1.763 | 0.933–3.332 | 0.081 | 1.923 | 0.997–3.711 | 0.051 |
| Renal disease | 1.477 | 0.494–4.412 | 0.485 | 1.566 | 0.509–4.814 | 0.434 |
| Psychological disease | 1.349 | 0.693–2.627 | 0.378 | 0.910 | 0.445–1.861 | 0.797 |
| Neurological disease | 2.189 | 0.580–8.259 | 0.248 | 1.755 | 0.436–7.071 | 0.429 |
| Digestive disease | 3.536 | 1.551–8.062 | 0.003 | 2.867 | 1.220–6.738 | 0.016 |
| Endocrine disease | 1.750 | 1.085–2.822 | 0.022 | 1.335 | 0.814–2.188 | 0.252 |
| Eyes, ear, nose, throat disease | 6.122 | 2.403–15.597 | 0.000 | 6.753 | 2.593–17.582 | 0.000 |
| Other disease | 1.790 | 1.287–2.489 | 0.001 | 1.781 | 1.260–2.519 | 0.001 |
| Days between first positive and first negative | 1.054 | 1.036–1.071 | 0.000 | 1.044 | 1.026–1.062 | 0.000 |
| Treatment within 4 weeks of diagnosis | 2.241 | 1.822–2.755 | 0.000 | 2.279 | 1.774–2.928 | 0.000 |
| Satisfied with the treatment | ||||||
| Yes | 0.565 | 0.474–0.673 | 0.000 | 0.588 | 0.490–0.705 | 0.000 |
| Missing | 0.495 | 0.407–0.603 | 0.000 | 0.778 | 0.614–0.986 | 0.038 |
| Number of symptoms within 4 weeks of diagnosis | 1.092 | 1.068–1.117 | 0.000 | 1.083 | 1.056–1.110 | 0.000 |
- Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
Having at least three doses of vaccine was not associated with a lower risk of long COVID symptoms (1.105, 0.985–1.239, p = 0.088). Among patients who received at least three doses of vaccine, compared with patients who received the CoronaVac vaccine, there was no significant difference in the risk of long COVID in those who received BNT162b2 (0.924, 0.772–1.104, p = 0.383) or mixed (1.061, 0.791–1.424, p = 0.692) or other vaccines (0.738, 0.272–2.005, p = 0.552). Further, the data showed that vaccination after the infection increased the risk of reporting symptoms (1.470, 1.199–1.804, p < 0.001).
A wide range of pre-existing comorbidities were also associated with an increased risk of long COVID symptoms, including ophthalmology/otorhinolaryngology disease (6.753, 2.593–17.582, p < 0.001), digestive disease (2.867, 1.220–6.738, p = 0.016), respiratory disease (2.050, 1.324–3.172, p = 0.001), hyperlipidaemia (1.732, 1.347–2.228, p < 0.001), cardio-cerebrovascular disease (1.666, 1.145–2.423, p = 0.008).
Several risk factors in the acute stage of illness were also associated with the incidence of long COVID. Longer days of infection (1.044, 1.026–1.062, p < 0.001) and more symptoms (1.083, 1.056–1.110, p < 0.001) were associated with the increased risk of long COVID. Among symptoms reported by patients at consultation during acute stage of illness, four of them were significant associated with increased risk of long COVID (Supporting Information: Figure 1), which include chest tightness (1.425, 1.182–1.718, p < 0.001), headache (1.241, 1.057–1.456, p = 0.008), fatigue (1.221, 1.084–1.375, p = 0.001), and diarrhea (1.185, 1.011–1.389, p = 0.036). The risk was significantly decreased among patients who were satisfied with the treatments in the acute stage of illness (0.588, 0.490–0.705, p < 0.001).
3.5 Risk factors for individual long COVID symptoms
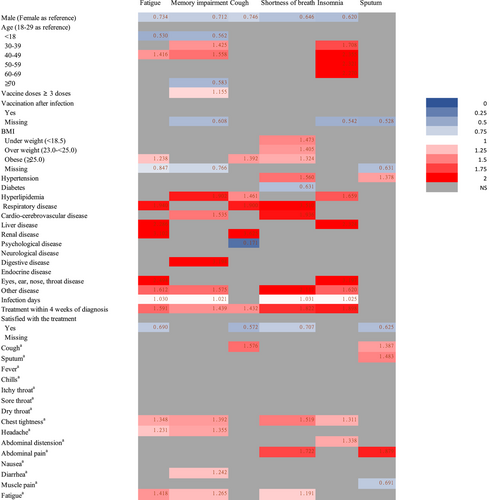
The association between risk factors and the six most common symptoms (fatigue, memory impairment, cough, shortness of breath, insomnia, and production of sputum) in long COVID patients are presented in Figure 3. Compared with female, male was significantly associated with a lower risk of developing fatigue, memory impairment, cough, shortness of breath, insomnia (all p < 0.05). Compared with adults aged 18–29 years, children/adolescent (<18 years) was associated with a lower risk of reporting fatigue (0.530, 0.345–0.814, p = 0.004) and memory impairment (0.562, 0.358–0.883, p = 0.012). Having ≥3 doses of vaccine was associated with an increased risk of reporting memory impairment (1.155, 1.008–1.325, p = 0.038). Compared with the normal range of BMI (18.5–23 kg/m2), obese patients were associated with a higher risk of fatigue (1.238, 1.012–1.515, p = 0.038), cough (1.392, 1.124–1.723, p = 0.002) and shortness of breath (1.324, 1.027–1.706, p = 0.030). Comorbidities were associated with the higher risks of common long COVID symptoms, for example, patients with hyperlipidemia were associated with increased risks of memory impairment (1.902, 1.453–2.490, p < 0.001), cough (1.461, 1.106–1.929, p = 0.008) and insomnia (1.659, 1.192–2.310, p = 0.003), and patients with respiratory disease had higher risks of fatigue (1.940, 1.290–2.917, p = 0.001), cough (1.900, 1.264–2.855, p = 0.002), and shortness of breath (3.510, 2.310–5.334, p < 0.001).
The association between all recorded symptoms in the acute stage and chronic stage are presented in Supporting Information: Figure 2. During the acute stage of illness, patients who reported fatigue (1.418, 1.238–1.623, p < 0.001), chest tightness (1.348, 1.109–1.639, p = 0.003), and headache (1.231, 1.034–1.465, p = 0.020) were associated with an increased risk of chronic fatigue in the long COVID stage. Having fatigue (1.265, 1.101–1.452, p = 0.001), chest tightness (1.392, 1.145–1.694, p = 0.001), headache (1.355, 1.138–1.614, p = 0.001), and diarrhea (1.242, 1.039–1.483, p = 0.017) within the acute infection were associated with a higher risk of memory impairment in long COVID stage. Having cough in the acute stage was associated with higher risks (1.576, 1.287–1.930, p < 0.001) of chronic cough in long COVID stage. Patients who were satisfied with the treatment during the acute stage of illness had lower risks of fatigue (0.690, 0.569–0.836, p < 0.001), cough (0.572, 0.469–0.696, p < 0.001), shortness of breath (0.707, 0.556–0.898, p = 0.005), and sputum production (0.625, 0.470–0.830, p = 0.001).
4 DISCUSSION
The results of our large-scale retrospective study provide important insights into the prevalence and risk factors associated with long COVID among individuals infected with the Omicron variant in Hong Kong. Our findings indicate that the prevalence of long COVID among nonhospitalized patients after 6–12 months of diagnosis is substantial, with over half (55.0%) of patients experiencing symptoms that persist after 6–12 months of diagnosis. Furthermore, a considerable burden of symptoms was found in this study, with 51.9% of patients experiencing a substantial mild to moderate burden of symptoms and over 10% of them being severely affected.
The prevalence of long COVID in this study was similar to a systematic review (45%) that included over 0.7 million patients from different countries and infected by different variants of concern (wild-type, Alpha, and Beta),3 but lower than a study from China, where the prevalence of long COVID infected by various variants (wild-type, Alpha, Beta, Delta, and Omicron) among mostly hospitalized patients was 90.4%.26 The SARS-CoV-2 subvariants were not detected for patients in our study, the majority of COVID-19 cases in the fifth wave of the Hong Kong outbreak were caused by Omicron BA.2 and related sublineages, the largest monophyletic lineage was BA.2.2, and only with limited detection of Omicron BA.1 and related sublineages and Delta AY.127.12 A systematic review indicated that the prevalence of Omicron-induced long COVID in 3 months (28.4%) was lower than other strains (wild-type: 52.1%, Alpha: 65.8%, Delta: 34.6%).27 The milder symptoms of Omicron may represent less damage in organs than the previous variants, resulting in a lower prevalence of long COVID.14 Compared with other studies with different follow-up period of long COVID in Omicron cases, the prevalence of long COVID during 6–12 months postacute infection in Hong Kong is higher, for example, 8.2% of COVID-19 survivors reported long COVID symptoms at 73 days after the infection in Eastern India, 10.9% at 2 months in United States, 4.5% at 4 weeks in United Kingdom, and 18.5% at 6 months in South Africa.14, 16-18
Our study revealed several important findings. First, the most common symptoms (>10%) reported by patients in our study are fatigue, memory impairment, cough, shortness of breath, insomnia, and the production of sputum. These symptoms are consistent with previous studies on long COVID symptoms in different populations infected with different variants.7, 17 These symptoms related to multiple organ systems, and Davis et al. well summarized the possible hypotheses for the pathogenesis of long COVID based on studies from the past 2 years, including immune dysregulation, with or without reactivation of underlying pathogens, autoimmunity and primed immune cells from molecular mimicry, occult viral persistence, microbiota dysbiosis, blood clotting and endothelial abnormalities, and dysfunctional neurological signaling.8 However, these hypotheses deserve further study to uncover the mechanisms behind them, and the findings will facilitate the effective treatment of this disease in the future.
Second, our results suggested that having three or more doses of vaccine was not lower the risk of long COVID compared with those who did not receive three doses. So far, the effect of vaccine on long COVID remains unclear, it may be influenced by various factors such as the SARS-CoV-2 variants, vaccine types, and time since vaccination. A report from the United Kingdom showed that double-vaccinated individuals with Omicron BA.1 were 50% less likely to experience long COVID than those with Delta, and triple-vaccinated participants had no significant difference.28 However, triple-vaccinated individuals had a higher incidence of long COVID after BA.2 infection than BA.1.28 In Hong Kong, the government has provided two vaccine types (inactivated virus vaccine: CoronaVac and mRNA vaccine: BNT162b2) to the community.24 In our study, the number of patients who received CoronaVac and BNT162b2 vaccines were similar among those who had received at least three doses. Some patients received a combination of the two vaccines. However, we found no significant difference in the risk of long COVID with different vaccine types. Although several studies indicated that BNT162b2 may have a protective effect against long COVID,29, 30 few studies have reported the protective effect of CoronaVac. Moreover, a study indicated that the impact of mRNA vaccination on long COVID symptoms varies among patients, with some experiencing relief (16.7%), worsening (21.4%), and others remaining unchanged.31 As the relationship between vaccination and long COVID is still controversial in different studies,32 further research is warranted to determine the role of different types of vaccine in long COVID and uncover the underlying mechanisms.
According to a meta-analysis study that involved different types of vaccine, the symptom changes among long COVID patients who received the vaccine after infection varied: 20.5% experienced worsening symptoms, 20.3% reported improvement, and 54.4% did not report any change.33 Some of the aggravated symptoms followed by vaccination were resolved within 1 week, but some may last for over months.34 Although we observed that patients who received vaccine after infection were more likely to report long COVID symptoms, further prospective studies are required to explore the impact of different types of vaccines on long COVID symptoms in patients who have been infected with COVID-19.
Third, in the acute stage of COVID-19, having more symptoms and presenting specific symptoms (fatigue/chest tightness/headache/diarrhea) were associated with an increased risk of long COVID. A study from Sudre et al. also reported experiencing more symptoms, having fatigue and headache during the 1st week of illness is one of the predictors of long COVID.2 We also identified several other risk factors associated with an increased incidence of long COVID symptoms, including female gender, age between 30 and 59 years, obesity, and the presence of comorbidities including ophthalmology/otorhinolaryngology disease, digestive disease, respiratory disease, hyperlipidemia, and cardio-cerebrovascular disease. Those risk factors were in line with the results from other studies.2, 5, 6, 17, 19 In addition, we found that compared to adults (18–29 years), children/adolescents had a lower risk of most common long COVID symptoms, which is consistent with other studies.35, 36 Although studies suggest that older age is a risk factor of long COVID,2 the elderly (>70 years) in our study were not associated with long COVID, it may due to hospitalized patients were excluded from this study and those in the older age group were more likely to be hospitalized. We found patient's satisfaction with the treatments was associated with a significantly lower risk of long COVID. The early treatments recommendation for COVID-19 in Hong Kong includes supportive therapy, symptomatic treatment, antiviral agents, and Chinese medicine.37 Patient's satisfaction with acute treatments could be linked to various factors, such as the early relief of symptoms, less severe acute symptoms, or a positive psychological state. These factors may be associated with a lower risk of developing long COVID. Therefore, additional prospective studies are needed to investigate the impact of these factors on the development of long COVID.
4.1 Strengths and limitations
A key strength of this study is its large sample size, which included individuals of all age groups. Also, we defined follow-up period 6–12 months after being diagnosed with COVID-19, which fulfilled the requirement of the WHO definition for long COVID. Another strength of our study is that all included subjects were nonhospitalized patients who were diagnosed with COVID-19 during the Omicron dominant outbreak in Hong Kong. To ensure the accuracy of our results, we excluded patients with new disease development or re-infection with COVID-19. Moreover, we accounted for co-variables in the acute stage of illness, such as treatment, treatment satisfaction, infection duration, and symptoms recorded during the first consultation.
Several limitations should be considered when interpreting the findings of this study. The retrospective nature of this study may introduce selection bias as all included patients were from the database of HKBU, which may not be representative of the general population. Moreover, recall bias could have affected the results, particularly in patients with neurocognitive symptoms. Additionally, the study was limited to Chinese Asians living in Hong Kong and may not be generalizable to other ethnicities or geographic locations. We also want to point out that this retrospective study was designed to report correlation but not causation, further prospective studies are needed to confirm potential causal relationships. The risk factors included in this study were not comprehensive, factors such as occupation, socioeconomic status, smoking, and other lifestyle factors that may influence the prevalence of long COVID were not included. While patients who were reinfected were excluded from the study, some patients who were reinfected may not have been tested, which may affect the prevalence of long COVID. In addition, this study did not consider the number of days between the last dose of vaccine and the follow-up visit, which is a potential confounding factor in the relationship between vaccines and long COVID. Besides, the study did not include a control group of people without a history of COVID-19, limiting the ability to confirm the association between the included symptoms and COVID-19. Finally, the study only included data up to 1 year after infection, and the long-term effects of long COVID remain to be studied in the future.
5 CONCLUSIONS
Our findings suggest Omicron caused long COVID in a significant proportion of infected individuals and induced a substantial impact on their normal life. The identified risk factors associated with long COVID symptoms can serve as a reference for the management of this condition, and guide the development of targeted interventions to protect patients infected with Omicron from long COVID. Further research is needed to confirm the impact of vaccination on the development of long COVID, to explore the underlying pathophysiological mechanisms of long COVID, and provide more evidence on the management of this condition. By shedding light on the prevalence and risk factors of long COVID after 6–12 months of Omicron infection, this large-scale study has the potential to inform global efforts to prevent and manage the long-term health implications of the Omicron-dominated COVID-19 outbreak, and other potential similar situation.
AUTHOR CONTRIBUTIONS
Zhaoxiang Bian developed the concept of this study. Zhaoxiang Bian and Chun Hoi Cheung led the study. Zhaoxiang Bian, Jingyuan Luo, Jialing Zhang, Chun Hoi Cheung, Hoi Ki Wong, and Hiu To Tang were responsible for conceptualization. Zhaoxiang Bian, Jingyuan Luo, and Jialing Zhang did data curation. Jingyuan Luo, Jialing Zhang, Hoi Ki Wong, and Hiu To Tang were responsible for the formal analysis. Jingyuan Luo, Hoi Ki Wong, and Hiu To Tang verified the underlying data. Zhaoxiang Bian, Jingyuan Luo, and Jialing Zhang wrote the original draft. All authors contributed to the review and editing. Jingyuan Luo, Jialing Zhang, Hoi Ki Wong, and Hiu To Tang had access to the raw data. All authors had access to all data in the study and the corresponding author had final responsibility for the decision to submit for publication.
ACKNOWLEDGMENTS
The authors wish to acknowledge patients who contributed to this study and the support from all medical staffs and research assistants from the School of Chinese Medicine, HKBU. Health@InnoHK Initiative Fund of the Hong Kong Special Administrative Region Government (ITC RC/IHK/4/7); Vincent and Lily Woo Foundation.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Individual, deidentified patient data can be made available at the request of investigators who propose to use the data for methodologically sound research. Data will be made available 6 months after article publication, with no end date. Requests for deidentified data should be made to the principal investigator.