Antibody response to the third dose of inactivated COVID-19 vaccine in people living with HIV (PLWH): A longitudinal cohort
Guang Zeng and Fei He contributed equally to this work.
Abstract
The immunogenicity induced by the third dose of inactivated coronavirus disease 2019 (COVID-19) vaccines in people living with HIV (PLWH) is unclear, and relevant literature is extremely scarce. It is important to add evidence on the humoral immune response induced by the third dose of inactivated COVID-19 vaccine in PLWH. We collected peripheral venous blood for spike receptor binding domain-protein specific immunoglobulin G (S-RBD-IgG) antibody tests at 28 days after the second dose (T1), 180 days after the second dose (T2) and 35 days after the third dose (T3) of inactivated COVID-19 vaccines in PLWH. The differences in S-RBD-IgG antibody levels and specific seroprevalence among T1, T2, and T3 time periods were analyzed, and the effects of age, vaccine brand, and CD4+T cell count on the levels and specific seroprevalence of S-RBD-IgG antibody induced by the third dose in PLWH were examined. The third dose of inactivated COVID-19 vaccines induced strong S-RBD-IgG antibody responses in PLWH. The levels and specific seroprevalence of S-RBD-IgG antibody were significantly higher than those at 28 and 180 days after the second dose and were not affected by vaccine brand or CD4+T cell count. Younger PLWH produced higher levels of S-RBD-IgG antibody. The third dose of inactivated COVID-19 vaccine showed good immunogenicity in PLWH. It is necessary to popularize the third dose in the PLWH population, especially PLWH who do not respond to two doses of inactivated COVID-19 vaccines. Meanwhile, the durability of the protection provided by the third dose in PLWH must be continuously monitored.
1 INTRODUCTION
Persistent human immunodeficiency virus (HIV) replication in people living with HIV (PLWH) leads to different degrees of immune deficiency and chronic inflammation, which may increase the risk of severe coronavirus disease 2019 (COVID-19).1-3 COVID-19 vaccines approved by the World Health Organization can effectively reduce the risk of severe disease and death from COVID-19, and can greatly benefit PLWH. It was reported that, by the end of 2021, there were approximately 38.4 million PLWH globally4; in China, there were approximately 1.045 million PLWH by the end of October 2022. The vaccine demands of such a large vulnerable population cannot be ignored; meanwhile, obtaining the best immunogenicity after vaccination is very important to mitigate the impact of the COVID-19 pandemic on the vulnerable PLWH population.
Two of the most widely used inactivated COVID-19 vaccines, BBIBP-CorV (Sinopharm) and CoronaVac (SinoVac), have facilitated global vaccine coverage and play an important role in the fight against COVID-19.5 Meanwhile, the immunogenicity of inactivated COVID-19 vaccines in immunodeficient populations, such as PLWH, has attracted wide attention. Previous studies have shown that due to different degrees of immunodeficiency, PLWH, especially those with CD4+T cell count <200 cells/µL, have lower antibody titers and seropositivity rates after vaccination with inactivated COVID-19 vaccines than healthy people.6-8 The specific antibodies induced by inactivated COVID-19 vaccines will gradually decrease over time.9, 10 Some studies have reported that the decline in the levels of antibodies induced by vaccines in PLWH may be faster than that in healthy people.11-13 It is likely that, before the third vaccination dose, most PLWH will lose protective antibodies, which will increase their risk of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection,14 especially by the more highly transmissible Omicron variant.15
Our previous study also reported a faster decline in spike receptor binding domain-protein specific immunoglobulin G (S-RBD-IgG) antibody induced by inactivated COVID-19 vaccines in PLWH compared with healthy controls, with only 16.3% (8/49) and 26.0% (13/50) of PLWH showing detectable levels of S-RBD-IgG antibody 6 months after two doses of the BBIBP-CorV vaccine or CoronaVac vaccine, respectively.16 This suggests that two doses of inactivated COVID-19 vaccines may not be sufficient to provide PLWH with long-term immunity against SARS-CoV-2. Meanwhile, the sequential emergence of SARS-CoV-2 variants with multiple mutations has raised concerns about the efficacy of two-dose regimens, especially against the Omicron variant, which has a high immune escape capacity.17 Therefore, it is necessary to prioritize booster immunization for PLWH to further consolidate the body's immunity against SARS-CoV-2.
The immunogenicity of the third dose of inactivated COVID-19 vaccine in PLWH has not been fully explored or evaluated. According to the literature, the third dose of the SARS-CoV-2 messenger RNA vaccine induced strong humoral and T-cell immune responses against SARS-CoV-2 in PLWH, with significantly higher antibody levels than the second dose. However, the neutralizing antibody response against the Omicron variant was always lower than that against the wild type. In addition, the humoral immune response of PLWH after the third dose was similar to or even higher than that of HIV-negative controls.18-20 A third dose of the BBIBP-CorV vaccine21, 22 or CoronaVac vaccine23 induced strong humoral and cellular immune responses and significantly increased antibody levels in healthy adults. However, for PLWH who have received two doses of inactivated COVID-19 vaccines, the evidence of antibody response after booster vaccination with inactivated COVID-19 vaccines is still unclear, and relevant literature is extremely lacking. To date, only Tan et al.24 have preliminarily evaluated early antibody responses to the third dose of an inactivated COVID-19 vaccine in PLWH receiving stable antiretroviral therapy (ART). Yan et al.25 reported that the seropositivity rate of specific IgG antibodies in PLWH after the third dose of inactivated COVID-19 vaccines was significantly lower than that in healthy controls. In this study, we describe the humoral immune responses of 65 PLWH after receiving a third dose of inactivated COVID-19 vaccine to provide a basis for further research.
2 MATERIALS AND METHODS
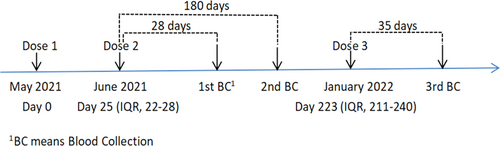
2.1 Study design and cohort
The cohort has been described previously.16 PLWH who were on routine follow-up and who had completed two doses of inactivated COVID-19 vaccines (BBIBP-CorV vaccine or CoronaVac vaccine) were recruited by convenience sampling. Inclusion criteria: (1) age 18–59 years old; (2) no history of SARS-CoV-2 infection nor history of close or indirect contact with people diagnosed with COVID-19; and (3) cooperation with blood collection, and follow-up. We collected peripheral venous blood for S-RBD-IgG antibody test at 28 days after the second dose (T1), 180 days after the second dose (T2) and 35 days after the third dose (T3) of inactivated COVID-19 vaccines in PLWH (Figure 1). At the end of the nearly 1-year cohort, a total of 65 PLWH have received three doses of inactivated COVID-19 vaccines and completed three blood collections for test successively, and they have not been infected with SARS-CoV-2. We identified n = 25/65 (38.5%) participants who received three doses of the BBIBP-CorV vaccine and n = 28/65 (43.1%) to three doses of the CoronaVac vaccine, whereas n = 12/65 (18.5%) participants who received three doses of hybird vaccines (Table 1). All the participants signed a written informed consent. This study complies with the requirements of the Declaration of Helsinki. All the collected information was anonymized and kept confidential.
| Characteristics | Statistic value |
|---|---|
| Males (n, %) | 62 (95.4%) |
| Age (median, IQR, range) | 34, 30–39, 23–59 |
| 18–39 | 50 (76.9%) |
| ≥40 | 15 (23.1%) |
| On stable ART before dose 1 (n, %) | 65 (100.0%) |
| Duration of ART before dose 1 (years, median, IQR) | 3.52, 1.54–5.13 |
| VL < 50 copies/mL before dose 1 (n, %) | 64 (98.5%) |
| CD4+T cell counts within 1 month before dose 3 (cells/µL, median, IQR, range) | 522, 417.5–674.5, 121–1068 |
| <350 cells/µL (n, %) | 9 (13.8%) |
| 350–500 cells/µL (n, %) | 20 (30.8%) |
| ≥500 cells/µL (n, %) | 36 (55.4%) |
| Vaccine types | - |
| 3 doses of the BBIBP-CorV vaccine (n, %) | 25 (38.5%) |
| 3 doses of the CoronaVac vaccine (n, %) | 28 (43.1%) |
| Hybrid vaccinea (n, %) | 12 (18.5%) |
| Days between dose 1 and dose 2 (median, IQR, range) | 25, 22–28, 21–48 |
| Days between dose 2 and dose 3 (median, IQR, range) | 195, 185.5–213, 174–306 |
- a Vaccination sequence: 2 doses of the BBIBP-CorV vaccine + 1 dose of the CoronaVac vaccine or 2 doses of the CoronaVac vaccine + 1 dose of the BBIBP-CorV vaccine or 1 dose of the BBIBP-CorV vaccine + 1 dose of the CoronaVac vaccine + 1 dose of the BBIBP-CorV vaccine or 1 dose of the BBIBP-CorV vaccine + 2 doses of the CoronaVac vaccine.
2.2 S-RBD-IgG antibody testing
Magnetic particle chemiluminescence kits (Shengxiang Biotechnology) were used to detect specific S-RBD-IgG antibodies in blood samples. The S-RBD-IgG antibody in the serum sample and the components in the reagent formed a complex of alkaline phosphatase-labeled antibody, S-RBD-IgG antibody, recombinant antigen, and magnetic powder. After the addition of the substrate, the alkaline phosphatase in the complex catalyzed the fluorescence of the substrate. The relative luminescence unit (RLU) of the complex to the substrate can indirectly reflect the level of the S-RBD-IgG antibody. The test result was expressed as the ratio of the sample luminescence value (S) to the cutoff (CO) (S/CO value), the S/CO value indirectly reflected the antibody level, and an S/CO value ≥ 1 was defined as S-RBD-IgG antibody seropositivity.
2.3 Statistical analysis
The data were organized using Microsoft Excel 2010, and statistical analysis was performed with IBM SPSS Statistics 25.0 (IBM Corp) and GraphPad Prism 8.4.2 (GraphPad Software). Baseline characteristics were reported as median with interquartile range (IQR). Enumeration data were described by constituent ratios. Antibody level and seropositivity rate were described as the median with IQR and rate with 95% confidence intervals (95% CI), respectively. Nonparametric test was used to compare the difference of median among groups, and the χ2 test or fisher exact test was used to compare the difference in the seropositivity rates among groups. For two-sided tests, a p-value of 0.05 or lower was considered statistically significant.
3 RESULTS
A total of 65 PLWH were included in the analysis. The main characteristics of participants are reported in Table 1. Briefly, 95.4% (62/65) were male, the median age was 34 years old (IQR, 30–39), and the 18–39 age group accounted for 76.9% (50/65). All PLWH were on stable ART before dose 1 and the median duration of ART were 3.52 years (IQR, 1.54–5.13), 98.5% (64/65) had HIV RNA viral load (VL) < 50 copies/mL before dose 1. The median CD4+T cell counts within 1 month before dose 3 were 522 cells/µL (IQR, 417.5–674.5), and 55.4% (36/65) had CD4+T cell counts above 500 cells/µL, two PLWH having CD4+T cell counts <200 cells/µL. The median time between dose 1 and dose 2 was 25 days (IQR, 22–28), While the median time from the second to the third dose was 195 days (IQR, 185.5–213) (Table 1).
3.1 Overall PLWH response to the third dose of COVID-19 vaccine
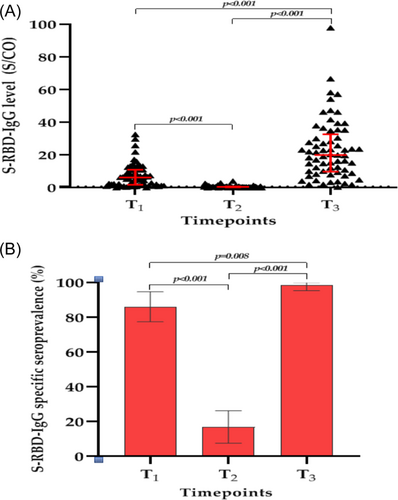
Regarding the S-RBD-IgG antibody level, the median S/CO values of S-RBD-IgG antibody in 65 PLWH decreased significantly from 6.3 (IQR, 1.8–11.0) at T1 to 0.5 (IQR, 0.3–0.8) at T2 (p < 0.001) but increased significantly from 0.5 (IQR, 0.3–0.8) at T2 to 20.0 (IQR, 9.8–32.7) at T3 (p < 0.001). Moreover, the median S/CO values of the S-RBD-IgG antibody at T3 was also significantly higher than those at T1 (p < 0.001) (Figure 2A). Regarding the S-RBD-IgG antibody specific seroprevalence, at T1, the specific seroprevalence was 86.2% (56/65, 95% CI: 77.5%, 94.8%), and nine PLWH had S-RBD-IgG antibody S/CO values below the lower limit of detection. At T2, the antibody specific seroprevalence decreased to 16.9% (11/65, 95% CI: 7.6%, 26.3%) (p < 0.001). However, the S-RBD-IgG antibody specific seroprevalence significantly increased from 16.9% (11/65) to 98.5% (64/65, 95% CI: 95.4%, 100.0%) at 35 days after the third dose (p < 0.001). It is worth noting that one PLWH coinfected with Hepatitis B virus (HBV) and whose CD4+T cell count remained below 350 cells/µL for years did not complete seroconversion, which we reported previously.26 Meanwhile, there was a statistically significant difference in the S-RBD-IgG antibody specific seroprevalence between T1 and T3 (p = 0.008) (Figure 2B).
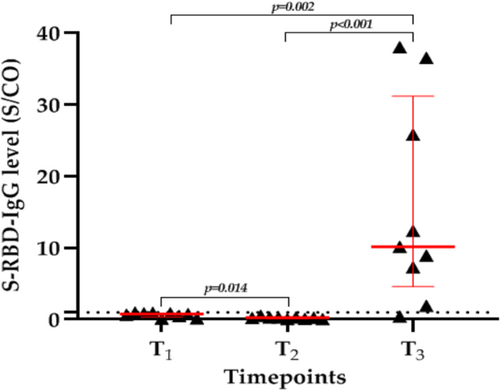
3.2 Response of PLWH with S-RBD-IgG antibody seronegativity at T1 to the third dose of COVID-19 vaccine
Nine PLWH failed to seroconvert after two doses of the inactivated COVID-19 vaccine. After the third dose, eight PLWH had high levels of S-RBD-IgG antibody, except for the one PLWH coinfected with HBV. The median S/CO values of S-RBD-IgG antibody increased significantly from 0.8 (IQR, 0.3–0.9) at T1 to 10.16 (IQR, 4.6–31.2) at T3 (p = 0.002) (Figure 3). These results suggest that PLWH who fail to seroconvert after two doses of inactivated COVID-19 vaccines are not true “nonresponders”; their immune memory has been successfully established and can last for at least 6 months but will need to be restimulated with antigen.
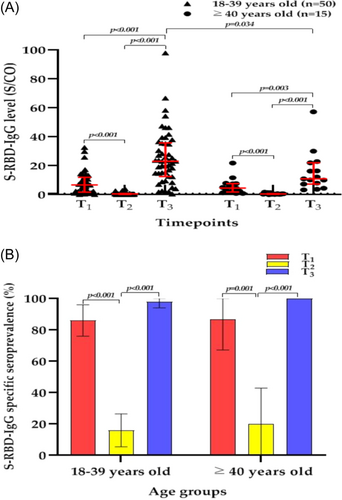
3.3 Age response to the third dose
We divided the 65 PLWH into two age groups: 18–39 and ≥40 years.25 We found that the median S/CO values of the S-RBD-IgG antibody in PLWH at T3 were significantly higher than those at T1 and T2 regardless of age group (all p < 0.05) (Figure 4A), and the specific seroprevalence of the S-RBD-IgG antibody at T3 were also significantly higher than those at T2 (all p < 0.05) (Figure 4B). However, at T3, the median S/CO values of S-RBD-IgG antibody in the 18–39-year group was significantly higher than that in the ≥40-year group (23.1 [IQR, 12.6–35.8] vs. 11.0 [IQR, 7.3–22.1], respectively; p = 0.34), although there was no significant difference in the S-RBD-IgG antibody specific seroprevalence between the two groups at T3.
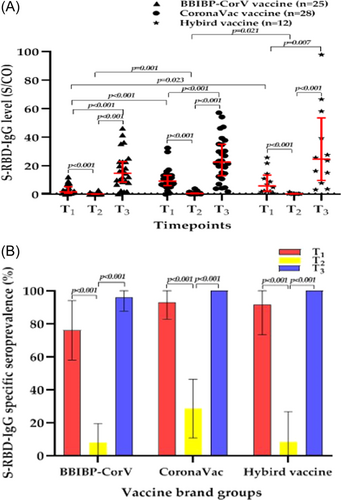
3.4 Response to the third dose of COVID-19 vaccine according to vaccine brand
The BBIBP-CorV vaccine and CoronaVac vaccine are both inactivated COVID-19 vaccines with the same technical route, although their manufacturers are different. We explored the differences in the levels and specific seroprevalence of the S-RBD-IgG antibody induced by different brands of inactivated COVID-19 vaccines in PLWH. We found that the S-RBD-IgG antibody levels of PLWH at T3 was significantly higher than those at T1 and T2, regardless of the vaccine brand (all p < 0.05) (Figure 5A). It is worth noting that the median S/CO values of the S-RBD-IgG antibody was 1.8 (IQR, 1.0–5.4) in PLWH vaccinated with two doses of the BBIBP-CorV vaccine, was significantly lower than the median S/CO values of 9.3 (IQR, 6.2–13.3) in PLWH vaccinated with two doses of the CoronaVac vaccine (p < 0.001); it was also significantly lower than the median S/CO values of 6.0 (IQR, 2.2–13.6) in PLWH vaccinated with two doses of hybrid vaccines (p = 0.023). However, there were no significant differences in the median S/CO values and specific seroprevalence of the S-RBD-IgG antibody among the groups at T3, which suggests that the antibody response induced by the third dose of inactivated COVID-19 vaccines in PLWH was not significantly affected by the vaccine brand (Figure 5B).
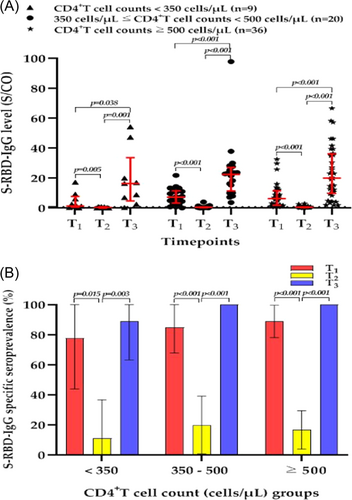
3.5 The CD4+T cell count in the response to the third dose of COVID-19 vaccine
We divided PLWH into three groups based on CD4+T cell count before the third dose: a <350-cells/µL group, a 350–500-cells/µL group, and a ≥500-cells/µL group. We found that the median S/CO values of the S-RBD-IgG antibody of PLWH at T3 were significantly higher than those at T1 and T2 regardless of the CD4+T cell count group (all p < 0.05) (Figure 6A), and the specific seroprevalence of the S-RBD-IgG antibody at T3 were also significantly higher than that at T2 (all p < 0.05) (Figure 6B). There were no significant differences in the median S/CO values and specific seroprevalence of S-RBD-IgG antibody among the CD4+T cell count groups at T3.
4 DISCUSSION
In this study, we found that the third dose of inactivated COVID-19 vaccines significantly enhanced antibody responses in PLWH treated with stable ART with well-suppressed VL, consistent with previous findings.24 Antibody levels and specific seroprevalence 35 days after the third dose of inactivated COVID-19 vaccines were significantly higher than those at 28 and 180 days after the second dose in PLWH, indicating that three doses of the vaccine resulted in a higher immune response than two doses in this population. More importantly, those PLWH who failed to complete seroconversion after the second dose all completed seroconversion and produced high levels of antibodies after the third dose, except for one who was coinfected with HBV. These findings indicate that two doses of inactivated COVID-19 vaccines can induce favorable immune memory against SARS-CoV-2 in PLWH that lasts for at least 6 months,27 and a strong humoral immune recall response was induced by the third dose. Meanwhile, Quast et al.28 pointed out that immune memory induced by an inactivated COVID-19 vaccine may provide ongoing protection against SARS-CoV-2, although further studies are required to confirm the correlation between immune memory and protection. Therefore, PLWH should receive a third dose as soon as possible, and the durability of protection provided by the third dose needs to be continuously monitored.
Age and sex appeared to influence the magnitude of humoral response in inactivated COVID-19 vaccine recipients, with younger recipients and women more likely to obtain higher titers and levels of S-RBD-IgG antibody.10, 29 In our cohort, only a small proportion (4.6% [3/65]) were women, owing to which we were unable to compare the effect of sex on the antibody response induced by the third dose in PLWH. We found that PLWH aged 18–39 years had significantly higher S-RBD-IgG antibody levels after the third dose than PLWH aged ≥40 years, although the difference in the specific seroprevalence between the two groups was nonsignificant. Yan et al.25 also reported that PLWH aged 18–39 years produced more effective neutralizing antibodies than PLWH aged ≥40 years after the third dose of inactivated COVID-19 vaccines, and age differences may affect the level and persistence of antibodies, thereby affecting the PLWH's benefit from the third dose.30
The BBIBP-CorV vaccine and CoronaVac vaccine are inactivated COVID-19 vaccines with similar technical routes. In this study, the S-RBD-IgG antibody level and specific seroprevalence of PLWH 28 days after two doses of the BBIBP-CorV vaccine were significantly lower than those of PLWH who received two doses of the CoronaVac vaccine; however, there was no significant difference in antibody level and specific seroprevalence at T3 induced by the three doses of the BBIBP-CorV vaccine, CoronaVac vaccine, or hybrid vaccines. This is a good news for PLWH who have access to any brand of inactivated COVID-19 vaccine nearby. Certainly, our sample size was small and more comprehensive data were needed to make a definitive judgment.
It is generally accepted in academia that the immunogenicity induced by a vaccine in PLWH is related to the individual's CD4+T cell count and VL.31 ART persistently suppresses HIV to undetectable levels in plasma and usually restores the CD4+T cell count, which are required for an effective immune response to the vaccine, to enhance the postvaccination immune response.32, 33 In our study, these PLWH were all receiving stable ART before the first dose with almost universally well-suppressed VL and high CD4+T cell count before the third dose. The third dose induced significantly higher antibody levels in PLWH than the second dose, regardless of the CD4+T cell count. Moreover, there were no significant differences in the S-RBD-IgG antibody levels or specific seroprevalence among the groups after the third dose. This suggests that PLWH with different CD4+T cell counts can benefit from the third dose of inactivated COVID-19 vaccine, which is very important. Unfortunately, PLWH with CD4+T cell count <200 cells/µL were underrepresented in this study. Previous studies have shown that PLWH with CD4+T cell count <200 cells/µL were poorly responsive to two doses of inactivated COVID-19 vaccines,7 and may benefit the most from the third dose. Future studies should include more of this subgroup as well as PLWH with poorly suppressed VL.
Recent studies have shown that a third dose of inactivated COVID-19 vaccine significantly increased neutralizing antibody levels in healthy adults; however, it may still be insufficient to counteract the Omicron variant, which is more likely to escape vaccine-induced immune protection.34, 35 For PLWH, especially those who fail to respond to the third dose, a fourth dose may be necessary. On December 14, 2022, the National Health Commission of China proposed to carry out a second booster dose immunization in PLWH and other immunocompromised populations.36 Moreover, on the premise of security, higher antigen titers per dose of vaccine are also worth considering. Cao et al.37 reported that healthy adults who received a high third dose of inactivated COVID-19 vaccines showed higher seropositivity rates and antibody responses against all SARS-CoV-2 variants; therefore, relevant studies should be considered in PLWH.
Our study has some limitations. First, the sample size is small. In a cohort lasting up to 1 year, the frequent local sporadic COVID-19 epidemic prevented participants from going to the designated acquired immunodeficiency syndrome treatment hospital for blood collection. Participants who missed blood collection in the previous stage could not participate in subsequent blood collection, and the strict experimental design resulted in only a small proportion of PLWH completing three blood collections. Second, loss to follow-up was more severe in our healthy control group, resulting in the inability to compare the differences in antibody responses induced by the third dose between the healthy control and PLWH groups. Third, the proportion of women was relatively small; therefore, we were unable to analyze the effect of sex on the antibody response induced by the third dose. Finally, at the time of our study initiation, the age of enrollment in PLWH was limited to 18–59 years according to national requirements; therefore, the antibody response induced by the third dose of inactivated COVID-19 vaccines in PLWH aged ≥60 years is still unclear and needs further in-depth study in the future.
5 CONCLUSION
The third dose of inactivated COVID-19 vaccine induced a strong S-RBD-IgG antibody response in PLWH on stable ART with well-suppressed VL. The antibody levels and specific seroprevalence were significantly higher after the third dose of inactivated COVID-19 vaccine than those at 28 and 180 days after the second dose, and were not affected by the vaccine brand or CD4+T cell count. Younger PLWH produced higher levels of S-RBD-IgG antibody. Our study highlights the necessity and importance of vaccination of the PLWH population with the third dose, especially those who do not respond to two doses of inactivated COVID-19 vaccines.
AUTHOR CONTRIBUTIONS
Guang Zeng and Fei He did the ideation, conceptualization, data and literature collection, statistical analysis, writing original draft and reviewing, and editing; Xiaomin Zhang did investigation, laboratory test, statistical analysis and funding; Guilian Li, Yongxia Gan and Chenli Zheng did investigation, data collection and editing; Xiaohui Wang did investigation, reviewing and funding; Jie Tang and Liumei Xu did investigation and follow-up; Jin Zhao did funding; Zhengrong Yang and Shuidong Feng did study supervision, study concept and design. Zhengrong Yang is guarantor for this study. The corresponding author attests that all listed authors meet authorship criteria. All authors critically reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank the study participants without whom these results could not be achieved. This study was funded by National Natural Science Foundation of China (grant number 81902097); Guangdong Provincial Basic and Applied Basic Research Foundation (grant number 2019B1515120003); and Shenzhen Municipal Science and Technology Innovation Committee Foundation (grant number JCYJ20180508152244835).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee of Shenzhen Center for Disease Control and Prevention (No. QS2021070043, date of approval: 10 August 2021).
Open Research
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author. The data are not publicly available according to the ethical committee decision on the conduct of this study.




