Combination of novel oncolytic herpesvirus with paclitaxel as an efficient strategy for breast cancer therapy
Xinyue Deng, Yinan Shen, and Ming Yi are contributed equally to this study.
Abstract
Background
New strategies are needed to improve the treatment of patients with breast cancer (BC). Oncolytic virotherapy is a promising new tool for cancer treatment but still has a limited overall durable antitumor response. A novel replicable recombinant oncolytic herpes simplex virus type 1 called VG161 has been developed and has demonstrated antitumor effects in several cancers. Here, we explored the efficacy and the antitumor immune response of VG161 cotreatment with paclitaxel (PTX) which as a novel oncolytic viral immunotherapy for BC.
Methods
The antitumor effect of VG161 and PTX was confirmed in a BC xenograft mouse model. The immunostimulatory pathways were tested by RNA-seq and the remodeling of tumor microenvironment was detected by Flow cytometry analysis or Immunohistochemistry. Pulmonary lesions were analyzed by the EMT6-Luc BC model.
Results
In this report, we demonstrate that VG161 can significantly represses BC growth and elicit a robust antitumor immune response in a mouse model. The effect is amplified when combined with PTX treatment. The antitumor effect is associated with the infiltration of lymphoid cells, including CD4+ T cells, CD8+ T cells, and NK cells (expressing TNF and IFN-γ), and myeloid cells, including macrophages, myeloid-derived suppressor cells, and dendritic cell cells. Additionally, VG161 cotreatment with PTX showed a significant reduction in BC lung metastasis, which may result from the enhanced CD4+ and CD8+ T cell-mediated responses.
Conclusions
The combination of PTX and VG161 is effective for repressing BC growth by inducing proinflammatory changes in the tumor microenvironment and reducing BC pulmonary metastasis. These data will provide a new strategy and valuable insight for oncolytic virus therapy applications in primary solid or metastatic BC tumors.
Abbreviations
-
- ALT
-
- alanine
-
- AST
-
- aspartate aminotransferase
-
- BC
-
- breast cancer
-
- BUN
-
- serum blood urea nitrogen
-
- Cr
-
- creatinine
-
- DCs
-
- dendritic cells
-
- GGT
-
- gamma-glutamyl transferase
-
- GM-CSF
-
- granulocyte-macrophage colony stimulating factor
-
- HSV-1
-
- herpes simplex virus type-1
-
- IFN-γ
-
- interferon gamma
-
- MHCII
-
- major histocompatibility complex class II molecules
-
- m-MDSCs
-
- myeloid-derived suppressor cells
-
- NKs
-
- natural killer cells
-
- OV
-
- oncolytic virus
-
- PD-L1
-
- programmed cell death ligand 1
-
- PTX
-
- paclitaxel
-
- TILs
-
- tumor-infiltrating lymphocytes
-
- TME
-
- tumor microenvironment
-
- TNF-α
-
- tumor necrosis factor alpha
-
- T-Vec
-
- talimogene laherparepvec
-
- UA
-
- uric acid
1 BACKGROUND
Breast cancer (BC) is the most common cancer in women worldwide. Although BC has been studied for many years, about 90% of patients still die from recurrence and metastasis.1, 2 Currently, surgery and chemotherapy are the main therapeutic options. Paclitaxel (PTX) is frequently used as the first-line treatment drug in BC, but innate and acquired resistance to PTX often compromises its applications.3 Therefore, new strategies are urgently needed to improve its efficacy and develop an alternative treatment for chemotherapy resistance.
Oncolytic viral therapy is emerging as a new therapeutic modality for cancer treatment, aiming for selective replication of the virus in cancer cells to induce host antitumor immunity. Multiple clinical studies have demonstrated its excellent safety and efficacy profiles. Several candidates, such as HF10, showed strong tumor killing activity through multiple mechanisms, including direct oncolysis. Talimogene laherparepvec (T-Vec) was developed as an oncolytic immunotherapy that expresses human granulocyte-macrophage colony stimulating factor to stimulate antitumor immune responses.4-6 However, recent studies have shown this type of therapy still has a limited overall durable antitumor response due to the generation of myeloid-suppressor cells to induce immune suppression.7 Therefore, the efficacy of oncolytic viruses (OVs) needs to be improved to allow this modality to enter mainstream cancer treatment.
A novel herpes simplex virus type-1 (HSV-1) OV named VG161 was identified as an oncolytic agent by our team and has entered phase I clinical trials in Australia and China. It carries interleukin (IL)−12, IL-15 with its receptor α unit (IL15RA), and a programmed cell death 1 ligand 1 (PD-L1) peptide blocker (PDL1B), leading to expression of these four immunomodulatory factors in the tumor microenvironment (TME). Chouljenko et al. have demonstrated that by combining potent oncolytic activity with a broad spectrum of immune-stimulatory payload genes, VG161 can induce robust oncolysis and stimulate a long-lasting antitumor immune response.8 VG161-mediated antitumor effects have been verified for human tumor models (LNCaP and U87) in nude mice, while it also induced strong antitumor immune responses in immune-competent mice (CT26 and A20).9, 10 Furthermore, the first human trial showed that single intra-tumoral injection (IT) in patients with advanced refractory solid tumors was safe and well-tolerated. As a relatively “cold” tumor, BC has poor tumor immunogenicity with a small amount of T cell infiltration but abundant levels of immunosuppressive cells.11, 12 Therefore, VG161 may provoke a strong immune response by altering the immune microenvironment of BC.
In this study, we focused on the efficacy of VG161 for the treatment of BC and explored cotreatment with PTX both in vivo and in vitro. We found that VG161 significantly inhibited BC cell proliferation and elicited a robust antitumor immune response. When administered together, VG161 could potentially prime the immune system to overcome primary resistance to PTX, while PTX could further enhance the immune activity of VG161. This combination therapy was well-tolerated with high response rates. This OV therapy can help shed light on novel clinical BC treatment methods.
2 METHODS
2.1 Cell culture
SK-BR-3, MCF-7, and MDA-MB-231 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). EMT-6 cells were grown in RPMI-1640 containing 10% FBS. All cells were cultured in a humidified 5% CO2 atmosphere at 37℃.
2.2 Cell viability assay
SK-BR-3, MDA-MB-231, MCF-7, and EMT-6 cells (10,000 cells/well) were seeded in 96-well plates and infected with VG161 at a multiplicity of infection (MOI) of 0, 0.01, 0.1, 1, or 10, or co-treated with 5 nM or 20 nM PTX, for the indicated times. Cells were then incubated with CCK-8 reagent (Beyotime, Shanghai, China) for 2 to 4 h. Absorbance values were measured at 450 nm using a plate reader (Bio-Rad).
2.3 VG161 and VG160 OV
Genes encoding human IL-12, IL-15, and IL-15 receptor alpha subunit isoform 1 (IL-15RA), and an expression cassette for secretable PD-L1 blocking peptide was conjugated to human IgG4 Fc (TF-Fc) were constructed into OV Herpes simplex virus type 1 (HSV-1), termed hVG161(VG161). The mVG161 was identical to VG161 apart from the substitution of mouse IL-12 for human IL-12 and the presence of a mouse-specific version of the PD-L1 blocker conjugated with mouse IgG1 Fc. In contrast, mVG160 was the backbone neither express mouse IL-12, IL-15, IL-15RA, nor the PD-L1 blocking peptide.
2.4 Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Sigma-Aldrich). The reverse transcription reaction was performed using a SuperScript RT-PCR kit (Thermo Fisher Scientific), and RT-qPCR was performed using SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biology) according to the manufacturer's protocol. mRNA levels were normalized to GAPDH level, and the results were performed with at least three independent experiments. The primer sequences are listed in Supporting Information: Table 1.
2.5 Enzyme-linked immunosorbent assays (ELISAs)
The levels of human IL-12p70, human IL-15/IL-15RA complex, or PD-L1 blocker in VG161-infected cell culture supernatants were quantified using the following ELISA kits: Human/Mouse IL-12p70 and IL-15 Human/Mouse ELISA kits were from Proteintech (Chicago, IL, USA), Human IgG4 ELISA kit was from Thermo Fisher Scientific and Mouse IgG1 ELISA kit was from Abcam.
Mice serum was analyzed for Interferon gamma (IFN-γ) and Tumor Necrosis Factor alpha (TNF-α) production using the IFN-γ and TNF-α Mouse DuoSet ELISA Kit (R&D Systems) according to the manufacturer's recommended procedures.
2.6 In vivo tumor models and lung metastasis model
All animal experiments were performed according to the proven procedures by the Institutional Animal Care and Use Committee at Zhejiang Laboratory Animal Center. BALB/c mice were implanted orthotopically with EMT-6 cells on both sides, then the right-side tumors were injected intratumorally with either PBS control or mVG161 for five consecutive days. The left side tumors were not treated. Subsequently, tumor size (length × width2/2) and mouse weight were recorded every 2 days. After 23 days of treatment, mice were killed and tumors were dissected and photographed. EMT6-Luc cells (50 × 104) were subcutaneously injected into the second mammary fat pad to establish an orthotopic transplantation model. The BALB/c mice were randomly divided into two or five groups: Vehicle, PTX, mVG161, PTX + mVG161, and mVG161 + PTX. Mice received mVG161 for three consecutive days and PTX every other day for two injections as indicated. After 19 days, lung metastasis was analyzed by an IVIS-100 imaging system (Xenogen). Mice were killed, the metastatic lung tissues were either fixed in picric acid solution and nodules were counted 72 h later or fixed in 4% paraformaldehyde and were detected in paraffin-embedded sections by hematoxylin and eosin (H&E) staining. After 120 h, immunohistochemical analysis of CD3+ and CD8+ T cells were performed on sections of the metastatic tumors.
2.7 RNA-seq and analysis
Tumors were collected 120 h after virus injection, then the total RNA was isolated using TRIzol reagent (Invitrogen). Library construction and sequencing were performed on a BGISEQ-500 platform by Wuhan Genomic Institution (www.genomics.org.cn, BGI). All reads were mapped to the mm10 mouse genome, and the uniquely mapped reads were subjected to RNA-seq data analysis using the Hierarchical Indexing for Spliced Alignment of Transcripts (HISAT) system.13 The raw data have been uploaded to the public database, BioProject ID: PRJNA949807.
2.8 Flow cytometry analysis
Tumors were harvested from mice and single-cell suspensions were made by incubating minced tumor at room temperature for 1 h in enzyme digestion buffer containing type V collagenase, type IV DNase, and type V Hyaluronidase. Cells were washed and incubated for 30 min on ice in staining buffer (Thermo Fisher Scientific) with different combinations of the following antibodies: Fixable Viability Dye eFluor780, PE-conjugated anti-CD8, AF488-conjugated anti-CD107a, PerCP-Cy5-5-conjugated anti-Ly-6C antibodies, and PE-conjugated anti-CD11c (Thermo Fisher Scientific); BUV395-conjugated anti-CD49b (BD Biosciences); PE-Cy7-conjugated anti-CD3, FITC-conjugated anti-CD11b, PE-Cy7-conjugated anti-Ly-6G, APC-conjugated anti-Ly-6C, eF450 anti-MHCII antibodies, BV785-conjugated anti-CD8, BV421-conjugated anti-CD45, and BV510-conjugated anti-CD45 (BioLegend). For intracellular staining of IFN-γ and TNF-α, cells pre-stained with antibodies targeting surface proteins were incubated with ×1 fixation/permeabilization buffer and subsequently stained with APC-conjugated anti-IFN-γ and FITC-conjugated anti-TNF-α antibodies (BioLegend) in ×1 permeabilization buffer (Thermo Fisher Scientific). FACS analysis was performed on a BD Fortessa X20.
2.9 Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Mouse tumor tissues were fixed in 4% (w/v) paraformaldehyde (PFA), dehydrated in an ethanol gradient, and embedded in paraffin. Samples were then cut into 3 μm sections using a Leica microtome. TUNEL assays were carried out according to the manufacturer's instructions (Roche Ltd.). Sections were analyzed using an inverted fluorescence microscope (Olympus).
2.10 Immunohistochemistry (IHC)
Mouse tumor tissues were fixed in 4% PFA, dehydrated in an ethanol gradient, and embedded in paraffin. Samples were then cut into 3 μm sections using a Leica microtome. Staining for caspase-3 (Thermo Fisher Scientific) was carried out according to the manufacturer's instructions.
2.11 Immunofluorescence staining and microscopy
Mouse tumor sections were mounted on glass slides and deparaffinized. For double immunofluorescence staining, sections were incubated in 3% skim milk in PBS-T for 30 min and then incubated overnight at room temperature with a combination of two primary antibodies. These combinations included rat monoclonal anti-CD3 (Proteintech), anti-CD4 (Proteintech), and anti-CD8 (Proteintech) antibodies. Sections were then incubated with a mixture of fluorophore-labeled secondary antibodies and counterstained with Hoechst. Immunofluorescence analysis of CD3+ and CD8+ T cells were performed on sections of the metastatic tumors. The images were analyzed using an inverted fluorescence microscope (Olympus).
2.12 Measurement of biochemical parameters
Whole blood was collected from the retro-orbital veins of mice using heparinized capillary tubes. After centrifugation, sera were collected and the levels of serum blood urea nitrogen (BUN), creatinine (Cr), uric acid (UA), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and alanine (ALT) were analyzed using the AU680 Chemistry System (Beckman Coulter).
2.13 Statistical analysis
Statistical analysis was performed with GraphPad Prism 8 (La Jolla). Data are expressed as the mean ± standard deviation (SD) and comparisons were made using a two-tailed Student's t-test or one-way analysis of variance. In all statistical tests, significance levels (p-values) are presented on the figures. *p < 0.05, **p < 0.01 and ***p < 0.001.
3 RESULTS
3.1 VG161 inhibits BC and lung metastasis
We first assessed the oncolytic capability of VG161 in BC cells. SK-BR-3, MDA-MB-231, MCF-7, and EMT-6 cells were infected with VG161 or mVG161 for 24, 48, and 72 h at MOIs ranging from 0.01 to 10. Cell viability was quantified by CCK-8 assays, which suggested that the VG161 virus inhibited viability in a dose-dependent manner (Figure 1A). Next, we detected the ability of VG161 to replicate in different tumor cell lines with plaque assays, and the result suggested that VG161 replicates effectively in the indicated cell lines (Figure 1B). In vivo experiments were used to further evaluate the efficacy of mVG161 after intratumoral inoculation in EMT6 tumor-bearing animals. BALB/c mice were implanted orthotopically with EMT-6 cells on both sides, then the right-side tumors were injected intratumorally with either PBS control or mVG161 for five consecutive days. The left side tumors were not treated. In animals treated with mVG161, the tumors were significantly regressed on the injected side with both 1 × 105 and 1 × 107 PFU virus. Interestingly, the non-injected side tumors were also regressed after 1 × 107 PFU mVG161 virus treatment (Figure 1C). Previous work by Chouljenko et al demonstrated that VG161 could induce a potent antitumor immune response in colon cancer.8 We hypothesized that this could also be applied to BC and that VG161 may induce antitumor immune responses that can inhibit the nontreated side tumors. Additionally, VG161 treatment resulted in significantly reduced lung metastasis of the tumors (Figure 1D−E). These data confirm that VG161 can repress BC cell proliferation and lung metastasis.
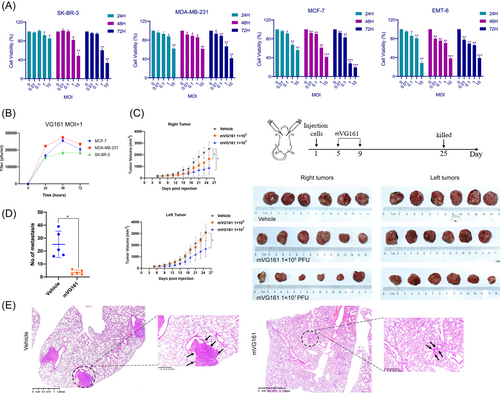
3.2 VG161 enhances the immune response in BC
VG161 is a genetically modified HSV consisting of IL-12, IL-15, IL-15RA, and a fusion protein (TF-Fc) capable of blocking PD-1/PD-L1 interactions. We first confirmed that the expression of human IL-12/IL-15 and PD-L1 blocker (IgG4) (Figure 2A–C) or mouse IL-12/IL15 and mIgG1 (Supporting Information: Figure 4) were strongly increased in the supernatants of VG161-infected BC cells. To further evaluate the antitumor immune response induced by VG161, we also used a dual tumor EMT model. We examined various immunostimulatory pathways in EMT6 tumors taken from BALB/c mice that were treated with either mVG161 on the right side or untreated on the left side using high-through sequencing of cDNA libraries (RNA-seq). Several immunostimulatory pathways, such as the TNF-α, NF-κB, and IL-17 signaling pathways, were activated in both the treated and untreated tumors (Figure 2D). RT-qPCR analyzed the expression of relevant genes in TNF and IL-17 pathway and the result illustrated the increased levels of those genes (Figure 2E). mVG161-injected right side and uninjected left side tumor sections were also stained and analyzed with IHC. The results displayed enrichment of CD3, CD4, and CD8 expression in mVG161-injected tumors compared with vehicle groups, suggesting the presence of tumor-infiltrating lymphocytes (TILs) in the TME (Figure 2H). Moreover, the antitumor immunity signature elicited by mVG161 was robust, as seen by increased production of IFN-γ and TNF-α (Figure 2F). Additionally, there were no abnormal changes noted in the liver and renal functional tests (Figure 2G), and the hematoxylin and eosin (H&E) staining of liver, heart, spleen, lungs and kidneys of mice were showed no significant damage (Supporting Information: Figure 5), suggesting a good safety profile in vivo. These findings further support the conclusion that VG161 can stimulate an antitumor immune response in BC in addition to oncolytic activity, with no apparent toxicity in the liver and kidney.

3.3 VG161 and PTX synergistically demonstrate greater tumor repression and tumor killing in vivo
Oncolytic HSV treatment is commonly combined with chemotherapy. This combination approach can achieve more efficient cancer cell killing than using either agent alone.14-16 To investigate this concept in our BC model, we combined mVG161 and PTX treatments and examined the antitumor efficiency in vivo and in vitro. Cells were pretreated with either VG161 or PTX for 24 h, followed by treatment with PTX or VG161 for 24, 48, or 72 h. CCK-8 assays showed that VG161 and PTX in combination could further suppress MCF-7 and MDA-MB-231 cell viabilities compared with either treatment alone. Notably, pretreatment with VG161 showed more significant effects than pretreatment with PTX (Figure 3A). We then confirmed our in vitro findings in tumor-bearing animals. EMT-6 cells were orthotopically implanted on both sides of BALB/c mice. Right side tumors were either injected intratumorally with mVG161 for three consecutive days followed by PTX treatment or pretreated with PTX followed by mVG161 infection. These results were consistent with our in vitro experiments showing that treating with mVG161 first had more efficient antitumor effects (Figure 3B). Next, to further verify whether the inserted genes carried by mVG161 can provide enhanced antitumor activity, we used mVG160 backbone virus as a positive control group. The result showed that PTX combined with mVG161 had stronger antitumor effect than PTX combined with mVG160, and the body weights of the mice in the six groups did not differ to a statistically significant extent (Figure 3C). In addition, we performed TUNEL assays and IHC analysis for the proapoptotic molecule caspase-3 in the right-side tumors. We found that the mVG161-PTX combination can induce greater apoptosis levels (Figure 3D–E). These results suggest that inducing an immune response with mVG161 before PTX treatment may enhance the effects of this chemotherapy to effectively fight BC.
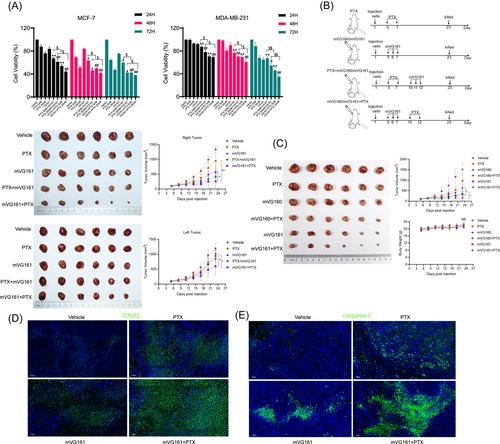
3.4 VG161 infection with PTX cotreatment reduces metastatic pulmonary lesions in the EMT-6 BC model
BC is a highly invasive disease and often metastasizes to the lungs. EMT6-Luc BC cells that were orthotopically implanted into BALB/c mice effectively metastasized to the lung and formed metastatic tumors. The number of metastatic tumors in the lungs was significantly reduced after mVG161 infection, as seen by bioluminescence ex vivo imaging and H&E staining. Metastatic pulmonary lesions were further decreased after cotreatment with PTX (Figure 4A–B). Additionally, IHC analysis showed enhanced infiltration of CD3+ and CD8+ T cells in the metastatic pulmonary lesions (Figure 4C). These findings indicate that VG161 in combination with PTX can decrease BC pulmonary metastasis and provide potential new therapeutic options for treating metastatic BC.
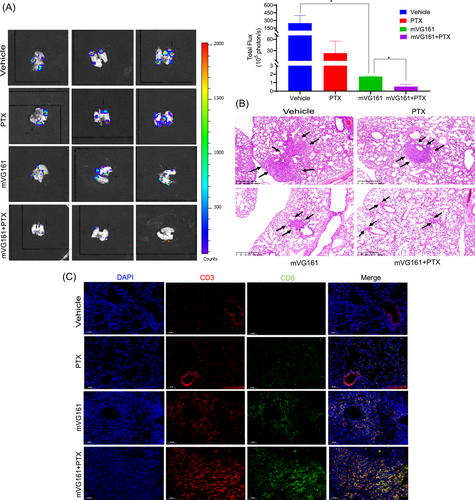
3.5 VG161 infection with PTX cotreatment greater alters the TME
To determine the antitumor immune response of mVG161 infection with PTX cotreatment, we utilized flow cytometry to analyze cells isolated from excised EMT-6 tumors of immunocompetent BALB/c mice treated with mVG161, PTX, or their combination. Infiltration of lymphoid cells, including CD8+ T cells and natural killer cells (NKs), as well as IFN-γ, TNF-α, and CD107a expression levels in these cells, increased following mVG161 injection. These observations were further enhanced following cotreatment with PTX. Myeloid cell infiltration, including macrophages, myeloid-derived suppressor cells (m-MDSCs), and dendritic cells (DCs), were also increased after this cotreatment (Figure 5A–B). These data show that VG161 infection followed by cotreatment with PTX can profoundly alter the TME composition to support a robust antitumor immune response in BC.
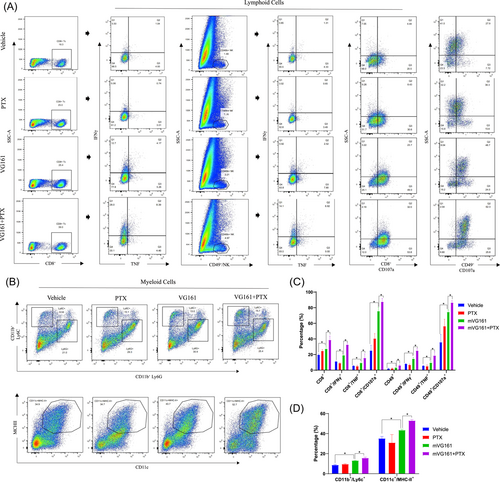
4 DISCUSSION
An oncolytic HSV is an ideal candidate for antitumor therapy because of its broad host range, tumor-selective viral distribution, and cancer cell-killing ability through induction of direct cell lysis and activation of the host immune response.17 Genetic engineering of herpesvirus vectors has been used for OV therapy, where cancer cells are killed through the direct oncolytic effect of the virus and its induction of host immunity.18, 19 VG161 is a genetically modified HSV with payloads consisting of IL-12, IL-15, IL-15RA, and a fusion protein (TF-Fc) capable of blocking PD-1/PD-L1 interactions. In this study, VG161 showed excellent antitumor activity in BC, with the combination with PTX inducing stronger cytotoxicity than with VG161 or PTX alone.
BC has not traditionally been considered a typical immunogenic tumor. OVs have been shown to convert immunologically “cold” tumors to “hot” tumors. However, locally injected OVs, such as HSV-1, may not sufficiently produce long-lasting antitumor immunity. VG161 was verified to play a much larger role in immunocompetent mice and elicit long-lasting antitumor immunity in colon cancer.8 This was also confirmed in this study in a BC xenograft mouse model. Mice treated with VG161 showed significant tumor regression both on the injected side and noninjected side. Notably, several immune-related signaling pathways were also activated on both sides. TNF-α and IFN-γ are proinflammatory cytokines that show potent antitumor activity and immunomodulatory capabilities, and can also induce major histocompatibility complex class II molecules (MHCII) to amplify CD4+ T cell activation and effector function.20, 21 In our experiments, VG161 markedly facilitated tumor infiltration of CD4+ and CD8+ T cells and promoted TNF-α and IFN-γ cytokine production. This suggests that VG161 can enhance immune activation and the immune-mediated clearance of tumor.
OV therapy is often used in combination with chemotherapy or radiotherapy to improve the efficacy of cancer treatments. Gemcitabine (GEM) is one of the first-line therapeutic agents used against pancreatic carcinoma. Esaki et al. has evaluated the synergistic antitumor effect of GEM in combination with the herpes simplex virus HF10, and found that the oncolytic activity of HF10 was enhanced when used in combination with GEM.22 Bourgeois-Daigneault et al. demonstrated that PTX cotreatment with oncolytic Rhabdovirus Maraba-MG1 could improve the efficacy better than with either treatment alone in suppression BC.15 These investigations have led us to further examine the most effective order of application between the OV and chemotherapy. We found that the combination of PTX with VG161 significantly inhibited BC tumor size, but applying the virus first had a more remarkable effect. Some current views believe that chemotherapeutics can increase virus replication and create a more favorable TME.23, 24 Here, we speculated that the pre-existing immunologic response by VG161 treatment may enhance the effects of conventional cytotoxic chemotherapy to eliminate cancer cells. Additionally, the destruction of tumor cells by chemotherapeutic agents may release tumor-associated antigens, and chemotherapy might act as a functional immunotherapy and further increase the immune response. What's more, VG161-PTX cotreatment can provide enhanced antitumor activity than VG160-PTX combination, this suggested that the inserted genes carried by VG161 inducing changes in tumor-infiltrating environment have more robust antitumor effect than VG160 backbone virus. BC frequently metastasizes to distant sites like the lungs,25 and patients with metastatic BC can often become resistant to current chemotherapy treatment methods.26 In our study, VG161 in combination with PTX exhibited a prominent effect in reducing BC pulmonary metastasis and facilitating tumor infiltration of CD3+ and CD8+ T cells in metastatic pulmonary lesions. This will provide a new strategy for treating metastatic BC.
TILs are widely used as a key indicator of the immune interactions between the host and tumor, and are also a potentially effective predictive biomarker of cancer immunotherapy.27, 28 “Effector” TILs, like CD4+ T helper 1 (Th1) cells, NK cells, CD8+ cytotoxic T cells, and DCs, are protective against tumor proliferation and were associated with pathological responses, while “suppressor” TIL subsets, like Fox3+, CD4+ Th2 cells, and MDSCs harbor immunosuppressive activity and promote tumor growth.27, 29-31 OVs are armed with transgenes that code for proteins that are beneficial to improving the overall antitumor efficiency. VG161 contains IL-12, IL-15, IL-15RA, and a PD-L1 blocker peptide, which all possess immune-stimulatory functions. OVs armed with IL-12 or IL-15 often showed better efficacy compared with the unarmed virus and could activate CD8+ T cells, NK cells, and DCs to exert antitumor activity.32-34 Notably, TILs are relevant for patient response to neoadjuvant chemotherapy in BC.35 We found higher levels of CD4+ T cells, CD8+ T cells, and NK cells in VG161-treated BC tissues, and these levels were further enhanced when the OV was combined with PTX treatment. Because TNF-α and IFN-γ signaling are key effector mechanisms for T and NK cell-mediated antitumor activity,36 we then examined the expression levels of IFN-γ, TNF-α, and CD107a to examine changes in CD8+ T cell and NK cell polyfunctionality. Again, improved effector functions of CD8+ T cells and NK cells were found after VG161 treatment, and the effects were further amplified when combined with PTX. Interestingly, Denkert et al. showed that when a tumor is infiltrated by T cells before beginning chemotherapy treatments, anthracycline neoadjuvant therapy is more effective for BC patients.35
Inflammatory DCs exhibit a CD11c+/CD11b+/Ly6C+ surface phenotype and play important roles in CD4+ T cells. VG161 co-treated with PTX increased CD11b+/Ly6C+ expression levels, which is consistent with work by Ma et al. showing that CD11c+/CD11b+/Ly6C+ TILs are essential for chemotherapy efficacy.37 The increased infiltration of myeloid DCs (defined as MHCII+/CD11c+) by VG161 or cotreatment with PTX may indicate polarization of MDSCs to an M1-like phenotype, which is considered an important component of immune of surveillance against tumors and can lead to enhanced antitumor immunity.38 The results indicate that VG161 co-treated with PTX could enhance the TME through T cell, NK cell, DC, and macrophage infiltration.
In conclusion, our research demonstrates that the combination of PTX and VG161 is effective for repressing BC growth by inducing proinflammatory changes in the TME and reducing BC pulmonary metastasis, as shown in (Figure 6) This study is of great importance and translating this finding into clinical treatment methods for BC will have a positive impact on patients.

AUTHOR CONTRIBUTIONS
Zhijun Dai and Chenfang Dong designed the research and supervised this study. Xinyue Deng, Yinan Shen, and Ming Yi designed and performed most of the research. Bin Zhao, Guansheng Zhong, Weiyang Lou, and Dixuan Xue performed data curation. Xinyue Deng wrote the manuscript; Qi Leng, Jun Ding, and Ronghua Zhao reviewed and edited the draft. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by National Key Research and Development Program of China (grant number 2019YFC1316000); Key project of Zhejiang Provincial Department of Science and Technology/Provincial Natural Science Foundation (grant number Z22H169268); Wu Jieping Medical Foundation (grant number 320.6750.2021-10-62); National Natural Science Foundation of China (grant number 82103044)We thank Figdraw for providing schematic image materials and J. Iacona, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




