Development of a CRISPR/Cas12a-recombinase polymerase amplification assay for visual and highly specific identification of the Congo Basin and West African strains of mpox virus
Xinggui Yang and Xiaoyan Zeng have equally contributed to this study.
Abstract
Human mpox is a zoonotic disease, similar to smallpox, caused by the mpox virus, which is further subdivided into Congo Basin and West African clades with different pathogenicity. In this study, a novel diagnostic protocol utilizing clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 12a nuclease (CRISPR/Cas12a)-mediated recombinase polymerase amplification (RPA) was developed to identify mpox in the Congo Basin and West Africa (CRISPR-RPA). Specific RPA primers targeting D14L and ATI were designed. CRISPR-RPA assay was performed using various target templates. In the designed CRISPR-RPA reaction system, the exponentially amplified RPA amplification products with a protospacer adjacent motif (PAM) site can locate the Cas12a/crRNA complex to its target regions, which successfully activates the CRISPR/Cas12a effector and achieves ultrafast trans-cleavage of a single-stranded DNA probe. The limit of detection for the CRISPR-RPA assay was 10 copies per reaction for D14L- and ATI-plasmids. No cross-reactivity was observed with non-mpox strains, confirming the high specificity of the CRISPR-RPA assay for distinguishing between the Congo Basin and West African mpox. The CRISPR-RPA assay can be completed within 45 min using real-time fluorescence readout. Moreover, the cleavage results were visualized under UV light or an imaging system, eliminating the need for a specialized apparatus. In summary, the developed CRISPR/RPA assay is a visual, rapid, sensitive, and highly specific detection technique that can be used as an attractive potential identification tool for Congo Basin and West African mpox in resource-limited laboratories.
1 INTRODUCTION
Human mpox is a zoonotic disease, similar to smallpox, caused by the mpox virus, an enveloped double-stranded DNA virus that is closely related to the variola virus.1, 2 Mpox belongs to the genus Orthopoxvirus in the family Poxviridae (subfamily Chordopoxvirinae) and is further subdivided into Congo Basin and West African clades.3 Generally, humans are infected with mpox via direct contact with the blood and body fluids.4, 5 Furthermore, human-to-human transmission mainly occurs through exposure to fomites and air droplets.2, 5 Mpox cases with smallpox symptoms in humans were first reported in Central Africa in 1970, whereas mpox was isolated from infected cynomolgus monkeys in 1958.6, 7 Its clinical symptoms are characterized by fever, rash, and lymphadenopathy, as well as other complications, such as pneumonia, encephalitis, and sight-threatening keratitis.2, 8 In previous studies, the virus clade circulating in the Congo Basin appeared to be more pathogenic, and the mortality rates reported ranged from 1% to 10% (the mortality rate associated with the West African clade was less than 3%).8-10 No specific vaccine is currently available against mpox, resulting in severe challenges to daily life in areas where mpox disease is common.9, 11 Thus, an ideal prevention and control strategy would be to rapidly identify the Congo Basin and West African mpox populations.
Currently, polymerase chain reaction (PCR) and PCR-based techniques, such as classical real-time PCR, are often used to detect and identify mpox infections.3, 12 Although these techniques have certain advantages in practical diagnostic work, they are time-consuming (~2 h) and require specialized instruments (e.g., real-time fluorescence detectors), making them difficult to use as readily available detection tools in resource-constrained laboratories.13 With the development of immunological diagnostic assays, various immunology-based techniques have been applied to detect the mpox, including enzyme-linked immunosorbent assay (ELISA) and immunofluorescence assay.14, 15 However, it must be admitted that they rely on expensive specific antibodies; thus, they are not readily available in resource-poor areas. Therefore, a simple, sensitive, low-cost, and highly specific assay is urgently required to detect mpox pathogens in populations. Presently, isothermal amplification technologies, such as recombinase polymerase amplification (RPA) and loop-mediated isothermal amplification (LAMP), seem to meet the above requirements because the amplification process only needs to be driven by thermostatic enzymes under isothermal condition.16, 17 The RPA assay, an attractive nucleic acid detection technique, has been devised and improved for the detection of various pathogens because of its simplicity, sensitivity, and reliability.18 However, amplicon verification in conventional RPA assays requires an additional agarose gel electrophoresis step, which increases the complexity of the detection process.11 Therefore, an advanced, intuitive, and simplified strategy for improving RPA assays is required.
Clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease (CRISPR/Cas) systems, which involve the bacterial adaptive immune system against foreign nucleic acids, have been widely designed and applied in the field of genome editing.19, 20 Currently, effector proteins of the CRISPR/Cas system, like Cas 9, Cas12a, Cas12b, and Cas13a, have been developed as highly specific detection tools for target pathogens.21, 22 In the CRISPR/Cas-mediated detection system, a single guide RNA (sgRNA) can navigate the Cas effector nuclease to its target site to form the target/sgRNA/Cas-nuclease complex, which then activates the collateral cleavage ability (i.e., trans-cleavage activity) to cleave specific single-stranded DNA/RNA (ssDNA/RNA) reporter molecules.23 Many CRISPR/Cas systems, such as CRISPR/Cas12 and CRISPR/Cas13, have been proven to be ultrasensitive in detecting various target sequences/pathogens because of their highly efficient collateral cleavage of ssDNA/RNA reporters.24, 25 Hence, integrating the CRISPR/Cas system with isothermal amplification techniques (including RPA and LAMP) is an ideal tool for rapidly identifying the Congo Basin and West African mpox.
However, these CRISPR/Cas-mediated isothermal amplification techniques, such as CRISPR/Cas12a-LAMP and -MCDA (multiple cross displacement amplification), involve multiple primer pairs (8 or 10 primers) and relatively high incubation temperatures (usually 63°C to 67°C).22, 23 Thus, a simplified protocol utilizing CRISPR/Cas12a-mediated RPA amplification to efficiently cleave ssDNA/RNA molecular probes was used as a specific sequence analysis tool.26 For example, the CRISPR/Cas12a system combined with an RPA assay, such as the DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR) assay, enables the rapid, sensitive, and specific detection of human papilloma virus (HPV) in human samples.20 Moreover, the Cas12a protein only requires a short crRNA (CRISPR RNA) sequence, which is more readily available than the Cas12b nuclease, which relies on a longer sgRNA sequence composed of crRNA and transactivating crRNA (tracrRNA).27 Meanwhile, visualization detection, an intuitive and easy-to-use verification method, was designed and applied to eliminate the use of special instruments and simplify the detection workflow.22 As a result, the CRISPR/Cas12a-mediated RPA amplification combined with visual detection and real-time fluorescence analysis is extremely attractive for early, rapid, and reliable detection, particularly in laboratories with limited resources.
In this study, a novel diagnostic platform utilizing CRISPR/Cas12a-mediated RPA amplification combined with real-time fluorescence analysis and visualization detection (UV light and/or imaging system) was developed for visual, rapid, sensitive, and highly specific discrimination of the Congo Basin and West African strains of mpox. The CRISPR-RPA assay targeting D14L (specific sequence of the Congo Basin mpox) and A-type inclusion body (ATI, specific sequence of the West African mpox) genes established in this study was evaluated using the corresponding plasmids, various pathogens, and simulated clinical samples.
2 MATERIALS AND METHODS
2.1 Materials and instruments
TwistAmp® Basic kits were purchased from TwistDx Ltd. Viral qEx-DNA/RNA and universal bacterial genomic DNA extraction kits were obtained from Xi'an Tianlong Science and Technology Co. Ltd. LbaCas12a nuclease (2.5 μg/μL) and Cas12a-ssDNA buffer Ⅱ (10×) were purchased from Novoprotein Co., Ltd. The PCR thermal cycler was purchased from Hangzhou Bioer Technology Co., Ltd. A ChemiDoc MP imaging system was obtained from Bio-Rad. A real-time quantitative fluorescence detector was obtained from Applied Biosystems. An ultramicro spectrophotometer was obtained from Thermo Fisher Scientific Co., Ltd.
2.2 Preparation of standard plasmids
The D14L- and ATI-plasmids used in this study were prepared and quantified by synthesizing D14L (GenBank accession no. KP849471.1, 19294-19944) and ATI (GenBank accession no. MT903346.1, 135527-135918) and subsequently cloned into the pUC57 vector. The entire preparation procedure was performed by Tsingke Biotechnology Co., Ltd. The original concentration of D14L and ATI-plasmids was 105 copies. Each initial plasmid concentration was serially diluted to obtain different diluents (105, 104, 103, 102, 101, 10°, and 10-1 copies).
2.3 Preparation of pseudo-viruses
Two pseudo-viruses containing the D14L sequence (GenBank accession No. KP849471.1, 19294-19944) and ATI sequence (GenBank accession no. MT903346.1, 135527-135918) were prepared by Sangon Biotech Co., Ltd. In brief, the preparation of the mpox pseudo-virus mainly included two steps: preparation of the overexpressed adenovirus vector and adenovirus packaging. First, the pUCm-T vector was digested with restriction endonucleases (NotI and KpnI) and recovered to obtain a linear vector by gel DNA purification (cat. B518743). The linear vector was transformed into DH5α competent cells (cat. CB101-02) for culture (37°C, 12~16 h) after being connected to the target fragment by homologous recombination or T4 DNA ligase. After successfully constructing shuttle plasmids, recombinant plasmids of high-purity adenoviral vectors were prepared using Escherichiacoli BJ5183 competent cells containing the adenoviral skeleton plasmid pAdeasy-1. After the recombinant plasmid was linearized using the PacI endonuclease, the linear plasmids were transfected into 293A cells using the transfection reagents. Finally, the target pseudo-viruses were obtained by amplification, purification, and quality detection. These pseudo-viruses were used to prepare simulated clinical samples to evaluate the applicability of the CRISPR-RPA assay. DNA templates were extracted from the simulated samples using viral qEx-DNA/RNA extraction kits (Xi'an Tianlong Science and Technology Co., Ltd). Nucleic acid concentrations were quantified using an ultramicro spectrophotometer (Thermo Fisher Scientific Co., Ltd).
2.4 Design of RPA primers and crRNA sequence
To obtain better amplification efficiency, RPA primers typically contain 30–36 nucleotides, and the GC content ranges from 40% to 60%. Moreover, the ideal size of RPA amplicons should be between 100 and 200 bp because the product length directly affects the amplification speed and sensitivity of the RPA reaction system. Based on the above design principles, five sets of specific RPA primers, including forward and reverse primers, were designed to target the D14L (GenBank accession no. KP849471.1, 19294-19944) and ATI genes (GenBank accession no. MT903346.1, 135527-135918) using Primer 3 Input software (version 0.4.0). Other details of primer design and screening were performed according to the design guidelines of the TwistAmp® Basic kit (http://www.twistdx.co.uk/images/uploads/docs/Appendix.pdf). All designed RPA primers were validated and analyzed to lower the possibility of nonspecific amplification of the sequence itself or with non-mpox strains using Basic Local Alignment Search Tool (BLAST) software. These primers were synthesized to HLPC-purified grade by Tianyi-Huiyuan Biotech Co., Ltd. Target sequences were amplified to further screen for optimal amplification primers, and the RPA amplicons were validated by 1.5% agarose gel electrophoresis (Supporting Information: Figure S1). In the current study, the target region amplified using the optimal D14L and ATI primers included a self-owned protospacer adjacent motif (PAM) site (TTTG). Typically, crRNAs consist of a scaffold sequence that directly forms a stem-loop structure (approximately 21 nt) and a spacer sequence that complements the target sequence (approximately 20 nt).23 According to previously reported design principles of crRNA sequences,23, 28 D14L- and ATI-crRNA sequences based on the PAM sites on the forward and reverse chains of the target templates were designed in our study. The locations of the RPA primers, PAM sites, and crRNA sequence-binding regions are shown in Supporting Information: Figure S2. Detailed information on the primers, crRNA sequences, and ssDNA reporters (including sequence, modification, and length) is provided in Supporting Information: Table S1.
2.5 RPA preamplification
The RPA reaction mixture was performed in a 50-µL reaction system using the commercial TwistAmp basic kits. The master mix (47.5 µL) consisted of 29.5 µL of rehydration buffer, 3 µL of each primer (forward and reverse primers, 10 μM), and an appropriate amount of nuclease-free water (11 µL for standard plasmids and other target templates, and 7 µL for simulated clinical samples) were added to a 0.2-mL reaction tube containing freeze-dried enzyme pellet and mixed by gentle pipetting. One microliter of standard plasmids, genomic templates of pure culture, or 5 µL of DNA template from simulated clinical samples were also added to the reaction tube. Then, 2.5 µL of magnesium acetate (280 mM) were pipetted into the tube lid, and the reaction tube was subsequently closed and centrifuged to mix the reaction system using a mini-spin centrifuge. The premixed tubes were immediately incubated at 39°C for 20 min. In the CRISPR-RPA assay, 1 µL of D14L- and ATI-plasmids (105 copies) were used as positive controls, 1 µL of genomic DNA from Mycobacterium tuberculosis was used as negative control, and 1 µL of nuclease-free water was used as blank control (BC). Moreover, a positive control from the RPA Basic Kit was used as an internal control to monitor the RPA reaction, and the RPA amplicons were validated using 1.5% agarose gel electrophoresis.
2.6 CRISPR/Cas12a-mediated trans-cleavage reaction
Firstly, 2.2 µL LbaCas12a nucleases were pre-incubated with 2.8 µL crRNAs (10 µM) in 1 × Cas12a-ssDNA buffer Ⅱ (500 mM NaCI, 20 mM sodium acetate, 0.1 mM EDTA, 0.1 mM TCEP, 50% glycerol, pH 6.0) at 37°C for 30 min to form the Cas12a/crRNA ribonucleoprotein (RNP) complexes. The RNP complexes were stored at 4°C before use (usually no more than 12 h). The CRISPR/Cas12a-mediated trans-cleavage reaction mixture (30 µL) consisted of: 15 µL of 2× ssDNA buffer Ⅱ (500 mM NaCI, 20 mM sodium acetate, 0.1 mM EDTA, 0.1 mM TCEP, 50% glycerol, pH 6.0), 1 µL of ssDNA-fluorescent quencher (ssDNA-FQ) reporter (5′-FAM-TTATTATT-BHQ1-3′, 100 μM), 6 µL of Cas12a/crRNA complexes, 4 µL of RPA preamplification products, and 4 µL of nuclease-free water. The reaction mixture was gently pipetted and centrifuged in a 0.2-mL PCR reaction tube. The premixed tubes were immediately incubated at 37°C for 30 min. Finally, the results of the CRISPR/Cas12a-mediated trans-cleavage of ssDNA reporter molecules were reported using a real-time fluorescence readout and visualization detection (UV light and imaging system).
2.7 Optimization of CRISPR/Cas2a-mediated trans-cleavage time
To achieve visual discrimination between the Congo Basin and West African mpox strains, CRISPR/Cas12a-mediated trans-cleavage times for visual detection were optimized using different incubation times ranging from 5 to 30 min at 5-min intervals. One microliter of serial dilutions of D14L- and ATI-plasmids (105, 104, 103, 102, 101, 10°, and 10−1 copies) were used as the amplification template for the Congo Basin and West Africa-CRISPR-RPA assays. The trans-cleavage results were visualized under UV light and an imaging system, and tests were performed according to RPA preamplification and the CRISPR/Cas12a-mediated trans-cleavage reaction.
2.8 Analytical sensitivity of the CRISPR-RPA assay
In this report, the analytical sensitivity of CRISPR-RPA for identifying Congo Basin and West African mpox strains was tested according to the CRISPR-RPA reaction. One microliter of serial dilutions of D14L- and ATI-plasmids (105, 104, 103, 102, 101, 10°, and 10−1 copies) was used as the amplification template. The results were monitored using a real-time fluorescence readout and visualization detection (UV light and imaging system), and the experiment was repeated more than three times.
2.9 Analytical specificity of the CRISPR-RPA assay
To evaluate the accuracy of CRISPR-RPA in discriminating between Congo Basin and West African mpox strains, a specificity assay was performed according to the CRISPR-RPA reaction. Here, a total of 29 nucleic acids extracted from different pathogens, including 21 viruses and 8 bacterial strains, were used as amplification templates. Details of the names and sources of the pathogens used in this study are shown in Table 1. Amplification templates from viral strains were prepared using viral qEx-DNA/RNA extraction kits, and bacterial DNA templates were extracted using universal bacterial genomic DNA extraction kits according to the manufacturer's instructions. One microliter of the target template (including standard plasmids and DNA templates from pseudo-viruses) was detected using the CRISPR-RPA assay, and the results were reported using a real-time fluorescence detector and visualization detection (UV light and imaging system). All tests were performed in triplicates.
| Pathogens/templates | Source of strains | No. of strains |
|---|---|---|
| Congo Basin mpox (D14L-plasmids) | Synthesized by Tsingke Biotech (Beijing, China) | 1 |
| Congo Basin mpox (pseudo-virus) | Synthesized by Sangon Biotech (Shanghai, China) | 1 |
| West African mpox (ATI-plasmids) | Synthesized by Tsingke Biotech (Beijing, China) | 1 |
| West African mpox (pseudo-virus) | Synthesized by Sangon Biotech (Shanghai, China) | 1 |
| Herpes simplex virus | CHCIP | 1 |
| Herpesvirus simiae (B virus) | CHCIP | 1 |
| Herpes zoster virus | CHCIP | 1 |
| Sendai virus | CHCIP | 1 |
| Influenza A virus | CHCIP | 1 |
| Influenza B virus | CHCIP | 1 |
| Vesicular stomatitis virus | CHCIP | 1 |
| Epstein–Barr virus | CHCIP | 1 |
| Measles virus | CHCIP | 1 |
| Respiratory syncytial virus | CHCIP | 1 |
| Parainfluenza virus | CHCIP | 1 |
| Adenovirus | CHCIP | 1 |
| Coxsackievirus A16 | CHCIP | 1 |
| Dengue virus | CHCIP | 1 |
| Rubella virus | CHCIP | 1 |
| Human rhinovirus | CHCIP | 1 |
| Parvovirus | CHCIP | 1 |
| Brucella melitensis | GZCDC | 1 |
| Bacillus anthracis | GZCDC | 1 |
| Klebsiella Pneumoniae | GZCDC | 1 |
| Staphylococcus aureus | GZCDC | 1 |
| Streptococcus pneumoniae | GZCDC | 1 |
| Mycobacterium tuberculosis | GZCDC | 1 |
| Mycobacterium leprae | GZCDC | 1 |
| Haemophilus influenzae | GZCDC | 1 |
| Total | 29 |
- Abbreviations: CHCIP, Children's Hospital Capital Institute of Pediatrics; GZCDC, Guizhou Provincial Center for Disease Control and Prevention.
2.10 Evaluation of the feasibility of CRISPR-RPA assay for the identification of simulated clinical samples
To evaluate the applicability of the Congo Basin and West Africa-CRISPR-RPA tests for actual detection, 62 simulated samples were prepared by constructing D14L-Congo Basin and ATI-West African pseudo-viruses. In other words, 62 throat swabs collected from healthy populations were wholly immersed in different sampling tubes containing 200-µL sterilized virus preservation solution (DaAn Gene Co., Ltd). These tubes were then randomly divided into two groups (i.e., groups 1 and 2), and D14L- and ATI-pseudo-viruses (approximately 101 to 105 copies) were randomly added to groups 1 and 2, respectively. Moreover, 32 additional throat swabs collected from healthy individuals were used as negative controls (without pseudo-viruses). Subsequently, the DNA templates from all simulated samples examined in the current study were prepared using the viral qEx-DNA/RNA extraction kits, and the extraction steps were performed using the automatic nucleic acid extractor (GeneRotex96) according to the manufacturer's guidelines (Xi'an Tianlong Science & Technology Co., Ltd). Five microliters of genomic DNA from the simulated samples were used as an amplification template, and the results were reported using real-time fluorescence detection and visualization detection (UV light and imaging system). All the samples were subjected to DNA sequencing (Tsingke Biotechnology Co., Ltd), and the sequencing results were compared and analyzed using the CRISPR-RPA assay.
3 RESULTS
3.1 Design of the CRISPR-RPA protocol
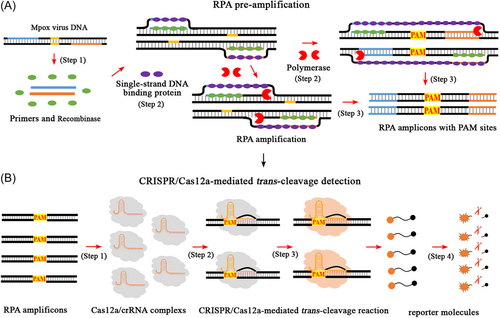
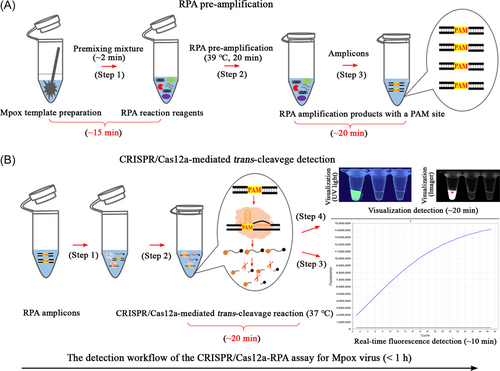
The CRISPR-RPA reaction system shown in Figure 1 mainly involves RPA preamplification and CRISPR/Cas12a-mediated trans-cutting detection. Two specific RPA primers (forward and reverse), which covered mpox templates with a self-owned PAM site (TTTG), were designed and synthesized (Figure 1A, step 1). These primers trigger RPA amplification (a PCR-like reaction) and exponentially synthesize amplicons with PAM sites (TTTG) driven by recombinase, polymerase, and single-strand DNA-binding proteins (Figure 1A, steps 2 and 3). In CRISPR/Cas12a-mediated trans-cleavage assays, specific PAM sites in the RPA amplicons can locate the preincubated Cas12a/crRNA complexes to their target sites to form Cas12a/crRNA/target conjugates and activate the collateral cleavage activity of the CRISPR/Cas12a effector (Figure 1B, steps 1, 2, and 3). The CRISPR-Cas12a/crRNA complexes then bind to the guide complementary RPA amplicons, thereby enabling the ultrafast cleavage of ssDNA reporter molecules (FAM-TTATTATT-BHQ1) within an extremely short time (Figure 1B, step 4). As a result, cutting the ssDNA probe released BHQ1, allowing the fluorophore to fluoresce (Figure 1B, step 4). In the practical tests, the entire workflow of the CRISPR-RPA assay included mpox template preparation (Figure 2A, step 1), RPA preamplification (Figure 2A, steps 2 and 3), and CRISPR/Cas12a-mediated trans-cleavage detection (Figure 2B, steps 1 and 2). In addition, the fluorescence signal emitted by the CRISPR-RPA detection system can be collected using a real-time fluorescence detector (Figure 2B, step 3) and visualized under UV light and/or using an imaging system (Figure 2B, step 4).
3.2 Assessment of the CRISPR-RPA assay
Confirmation tests were performed to demonstrate the effectiveness of the CRISPR-RPA assay in detecting Congo Basin and West African strains of mpox. In the reaction system of the CRISPR-RPA assay, one microliter of D14L- and ATI-plasmids (105 copies) was used as amplification template. First, the RPA preamplification products were validated using 1.5% agarose gel electrophoresis (Supporting Information: Figures S3A and S4A). In CRISPR/Cas12a-mediated trans-cleavage detection, bright fluorescence was observed with the naked eye under UV light (Supporting Information: Figures S3B and S4B) and with an imaging system (Supporting Information: Figures S3C and S4C), and a large number of fluorescence signals were collected using a real-time fluorescence detector (Supporting Information: Figures S3D and S4D). In contrast, no fluorescence signals were observed in the negative, blank, or RPA-positive controls (Supporting Information: Figures S3 and S4).
3.3 Optimal trans-cleavage time of the CRISPR-RPA assay for visualization
To explore the optimal trans-cleavage time of the CRISPR-RPA assay for visualization, Congo Basin and West Africa-CRISPR-RPA assays were performed using different reaction times (5, 10, 15, 20, 25, and 30 min). Optimization tests of Congo Basin- and West Africa-CRISPR-RPA assays were performed as previously described. As shown in Supporting Information: Tables S2 and S3, the lowest concentrations of D14L- and ATI-plasmids detected by the Congo Basin and West Africa-CRISPR-RPA assays were 10 copies per reaction at reaction times of 20, 25, and 30 min. Thus, the optimal cleavage time for the Congo Basin and West Africa-CRISPR-RPA assays for visual detection (UV light and imaging system) was 20 min.
3.4 Analytical sensitivity of the CRISPR-RPA assay
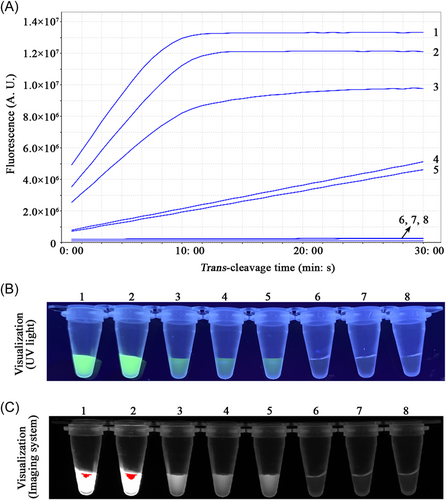
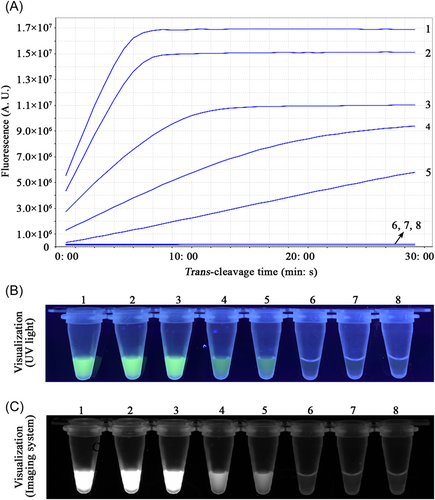
To evaluate the limit of detection of the CRISPR-RPA assays, sensitivity assays of the Congo Basin- and West Africa-CRISPR-RPA were performed by examining serial dilutions of standard D14L- and ATI-plasmids (105, 104, 103, 102, 101, 100, and 10-1 copies). In the current study, the lowest concentration of the standard plasmid detected by the Congo Basin- and West Africa-CRISPR-RPA assays was 10 copies per reaction (Figures 3 and 4). The verification results were consistent using real-time fluorescence analysis and visual detection (UV light and imaging system).
3.5 Analytical specificity of the CRISPR-RPA assay
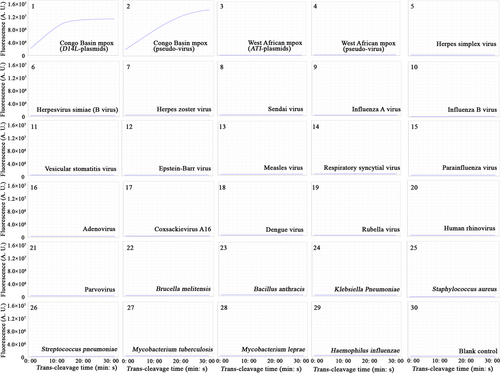
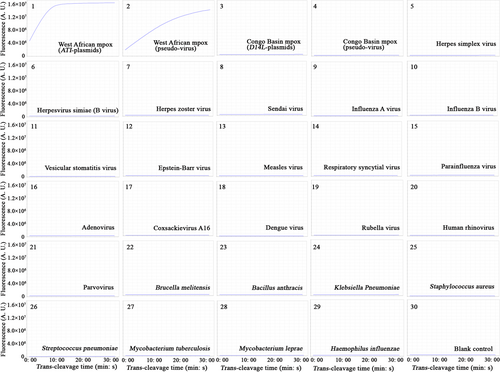
After repeated experiments, the Congo Basin-CRISPR-RPA assay detected the D14L-plasmid and Congo Basin mpox-D14L-pseudo-virus, and no cross-reaction occurred with the non-Congo Basin strains (Figure 5 and Supporting Information: S5). Similarly, the West Africa-CRISPR-RPA assay identified the corresponding ATI-plasmid and West African mpox-ATI-pseudo-virus and excluded all non-West African microbes (Figure 6 and Supporting Information: S6). In the mpox-CRISPR-RPA system, the results of real-time fluorescence and visualization detection (UV light and imaging system) were consistent in verifying the CRISPR/Cas12a-mediated trans-cleavage of ssDNA reporters. No cross-reactivity was observed when identifying the Congo Basin and West African mpox, confirming that the analytical specificity of the CRISPR-RPA assay was 100% (Figures 5 and 6, Supporting Information: Figures S5 and S6).
3.6 Applicability of the CRISPR-RPA assay to simulated specimens
A total of 94 throat swab specimens (namely, mpox, and no-mpox groups) were examined by Congo Basin- and West Africa-CRISPR-RPA, respectively. In the mpox group (62 samples), 25 simulated samples tested positive and 37 were negative by the Congo Basin-CRISPR-RPA test. Twenty-seven positive specimens and 35 negative samples were detected by the West Africa-CRISPR-RPA assay (Table 2). Thirty-two samples that did not contain any pseudo-virus (i.e., the no-mpox group) tested negative by the Congo Basin and West Africa-CRISPR-RPA assays (100%, 32/32). No cross-reactivity was detected between the samples tested as Congo Basin mpox-positive by the Congo Basin-CRISPR-RPA assay and specimens examined as West African mpox-positive by the West Africa-CRISPR-RPA assay (Table 2). The real-time fluorescence analysis and visualization detection (UV light and imaging system) used in this study were consistent with the validation of the CRISPR-RPA-mediated trans-cleavage of the ssDNA reporter. Moreover, the DNA sequencing results were consistent with those of the mpox-CRISPR-RPA assay for all samples tested in the current study. The entire workflow of the CRISPR-RPA assay was completed in less than 1 h, including DNA template preparation (approximately 15 min), RPA preamplification (approximately 20 min), CRISPR/Cas12a-mediated real-time fluorescence analysis (approximately 10 min), and visualization detection (approximately 20 min). These data demonstrate that the mpox-CRISPR-RPA assay is a visual, ultrafast, sensitive, and highly specific method for identifying the Congo Basin and West African mpox infections in human populations.
| Detection method | Mpox-group (n = 62) | |
|---|---|---|
| Congo Basin clade | West African clade | |
| DNA sequencing | 25 | 27 |
| Congo Basin-CRISPR-RPA-RTF | 25 | 0 |
| Congo Basin-CRISPR-RPA-VD | 25 | 0 |
| West Africa-CRISPR-RPA-RTF | 0 | 27 |
| West Africa-CRISPR-RPA-VD | 0 | 27 |
- Abbreviations: RTF, real-time fluorescence detection; VD, visual detection (UV light and imaging system).
4 DISCUSSION
To date, mpox infections mainly occur in resource-limited regions, such as the Congo Basin and West Africa, and mpox strains circulating in the Congo Basin appear to be more pathogenic to the human population.2, 7, 8 One of the reasons for the re-emergence of mpox transmission is closely related to the lack of detection capabilities.3 Hence, improving the ability to accurately discriminate between the Congo Basin and West Africa mpox is essential for rapid and reliable testing, especially in poorly resourced laboratories.
In 2012, a two-RNA structure that directed an endonuclease to cleave the target DNA in the CRISPR/Cas system was revealed as a landmark achievement in the field of gene editing.29 Subsequently, the CRISPR/Cas technique was successfully used for mammalian and human cell genome engineering in 2013.30, 31 CRISPR/Cas-based nucleic acid analysis techniques, as an attractive detection tool, have been recently developed and applied, such as classic DETECTR, a one-Hour Low-cost Multipurpose highly Efficient System (HOLMES), and Specific High-Sensitivity Enzymatic Reporter UnLOCKing (SHERLOCK) technologies.20, 25, 32 These technologies enable ultrasensitive and highly specific detection of target pathogens in a very short time (within 1 h), providing a new protocol for the diagnosis of clinical diseases, especially pandemics, like COVID-19.33 In particular, the CRISPR/Cas system combined with the isothermal amplification technique is considered an ideal diagnostic strategy because it avoids the reliance on thermocyclers, such as PCR-based assays.33
Currently, the CRISPR/Cas-LAMP and CRISPR/Cas-MCDA techniques have shown high sensitivity and specificity for the detection of target pathogens.23, 33 However, these methods mainly rely on specialized instruments (real-time fluorescence detectors) or multistep detection processes (preamplification, CRISPR/Cas-mediated trans-cleavage of ssDNA reporters, and label-based biosensor validation).23, 33 These requirements may render their application in resource-limited areas difficult. In addition, CRISPR/Cas12a-based detection techniques are more readily available because they only require a short crRNA sequence (approximately 41 nt) compared with the guide RNA required by the CRISPR/Cas12b system, which consists of crRNA and tracrRNA (approximately 111 nt).27 As a result, a novel diagnostic strategy that uses RPA amplification combined with a CRISPR/Cas12a system for visual, fast, sensitive, and highly specific discrimination of the Congo Basin and West African mpox was reported in this work.
Screening specific molecular targets is critical for accurate identification of the Congo Basin and West African mpox. In this study, the D14L and ATI sequences were used to design specific RPA primers because they have been proven to be ideal targets for identifying Congo Basin and West African mpox.7 These target genes were also found to be highly conserved in recently circulating mpox clades using BLAST software alignment. The CRISPR-RPA assay targeting the D14L and ATI sequences could accurately distinguish the Congo Basin from West African mpox using real-time fluorescence and visualization detection (UV light and imaging systems), confirming its effectiveness in distinguishing the Congo Basin from West African mpox (Supporting Information: Figures S3 and S4). The results of the CRISPR/Cas12a-based trans-cutting of the ssDNA reporter could be visualized under UV light, eliminating the need for specialized instruments (Figure 2). Visual detection is suitable for the simple, rapid, and specific detection of mpox infection, especially in basic laboratories with limited resources, because it avoids additional validation steps, such as the use of biosensors, and reduces testing costs.23 Importantly, the CRISPR/Cas12a-mediated trans-cleavage assays were able to exclude internal control of RPA preamplification in our assessment assay, thereby demonstrating its high specificity for identifying the target amplification fragment. (Supporting Information: Figures S3 and S4). Similarly, the CRISPR/Cas12a-crRNA system cannot recognize nontarget amplicons from primer dimers and spurious amplification products.22 This differential recognition performance is because nontarget fragments do not contain a PAM site that guides the CRISPR/Cas12a-crRNA complex to its target site and a target region that specifically binds to the spacer sequence in crRNA sequences. Moreover, isothermal amplification technologies, such as RPA, LAMP, and MCDA assays, are easy to produce carryover contamination because of the high-concentration amplification products generated during the incubation procedure.11, 34, 35 The CRISPR/Cas12a system can effectively cleave target amplicons to avoid false-positive results caused by carryover contamination.22
In the designed mpox-CRISPR/Cas12a-RPA system, the Cas12a/crRNA complex specifically recognizes its target region under the guidance of the PAM site (TTTG), which activates the collateral cleavage activity of the CRISPR/Cas12a system (Figure 1).23 Typically, a recognizable PAM site (TTTN) is required for the CRISPR/Cas12a system to activate collateral cleavage of the ssDNA reporter (trans-cleavage activity).22, 23 Currently, the specific PAM site (TTTN) required may be a limitation of CRISPR/Cas12a systems when the target amplification region does not contain a proper original PAM site.23, 33 Here, we designed an advanced protocol to overcome this limitation by modifying the PAM site (TTTT) at the 5ʹ end of the reverse primers (data not shown). Ideal schemes for engineered reverse primers were proven to accurately identify the Congo Basin and West African strains in the current study. In other words, CRISPR/Cas12a-based techniques, such as CRISPR/Cas12a-RPA, -LAMP, and -MCDA, can be used to detect any target sequence that does not contain specific PAM sites as long as preamplification assays are normally performed.
Although CRISPR/Cas12a system-based tests have been developed for the specific and sensitive detection of mpox, they could not identify Congo Basin or West African mpox.36-38 In addition, compared to that in the recently established MCDA-LFB test for identifying endemic clades of the Congo Basin and West African mpox, CRISPR/Cas12a-mediated revalidation of the amplicons could effectively avoid nonspecific amplification in the current study.39 In particular, the visual detection (UV light and imaging system) devised in our mpox-CRISPR-RPA detection system reduced the test costs associated with the additional use of biosensors in the MCDA-LFB assay. Although visual detection requires more time (approximately 20 min) than that of real-time fluorescence analysis (approximately 10 min), it is exceptionally suitable for laboratories in resource-poor areas (Figures 3 and 4; Supporting Information: Tables S2 and S3). Thus, both verification methods for the CRISPR-RPA assay can be used as complementary methods under different environmental conditions. The Congo Basin-and West Africa-CRISPR-RPA assays established in this study showed excellent detection sensitivity (10 copies per reaction), which is a critical factor for improving the detection rate of mpox infections in populations (Figures 3 and 4). Meanwhile, the mpox-CRISPR-RPA assay with high specificity could accurately identify target plasmids and pseudo-viruses from all pathogens tested in the current study, and no cross-reactivity was observed (Figures 5, 6,Supporting Information: S5 and S6). Although the CRISPR-RPA test accurately identified all the representative organisms used in the study, it was not involved in related pathogens, such as the eradicated variola virus. This limitation needs to be further verified if the virus is to re-emerge in the future.
Admittedly, a one-step assay, synchronous implementation of RPA preamplification, and CRISPR/Cas12a-mediated trans-cleavage in a single tube are optimal detection approaches. However, when the one-step mpox-CRISPR-RPA assay was performed in the current study, the detection efficiency was extremely low after testing several different reaction systems. The major reasons for this phenomenon are as follows: (i) both RPA amplification and the CRISPR/Cas12a system act on the same region of the target sequence; cis-cleavage of the mpox templates by the CRISPR/Cas12a system resulted in a lower concentration of mpox templates in the RPA reaction mixture, and (ii) when the trans-cleavage role of the CRISPR/Cas12a system was activated in the reaction mixture, the trans-cleavage of the specific single-chain RPA primers caused degradation of the RPA primers and affected the detection efficiency.40 Compared with the two-step mpox-CRISPR-RPA assay in our current study, the one-step method for identifying mpox infection can further simplify the test workflow and reduce detection time (~20 min). Hence, a one-step detection platform based on RPA amplification and the CRISPR/Cas12a system is highly anticipated to detect common causative agents or re-emerging infectious organisms by improving reaction mixtures in the near future.
In evaluating the practical detection capabilities of the mpox-CRISPR-RPA assay, as only a few cases of mpox are present in China, it was difficult to collect clinical specimens from patients with mpox. Thus, the D14L and ATI pseudo-viruses were used to prepare simulated clinical samples to evaluate the CRISPR-RPA assay and provide a competitive potential detection protocol for the possible emergence of a pandemic, such as COVID-19. However, 10 specimens were tested as negative by the Congo Basin- and West Africa-CRISPR-RPA methods in the mpox-group sample (16.13%, 10/62). This false-negative phenomenon may be because the nucleic acids concentration of the pseudo-virus contained in the specimens was lower than that of the detection limit of the CRISPR-RPA assay (namely, less than 10 copies/reaction). Nonetheless, the detection results of the CRISPR-RPA assay were consistent with those of the DNA sequencing analysis, indicating that the CRISPR-RPA assay has potential application value for identifying Congo Basin and West African mpox infections in populations. RPA pre-amplification and CRISPR/Cas12a-mediated trans-cutting of ssDNA reporter molecules can be performed in a heating apparatus that provides lower thermostat conditions, even in a thermostatic water bath. The entire detection workflow of the CRISPR-RPA assay was completed in <1 h. In addition, the single detection cost of the CRISPR-RPA assay is approximately 6.5 USD, including commercialized RPA amplification reagents and the Cas12a protein, making it highly accessible in resource-poor areas. These data confirmed that the CRISPR-RPA assay is a visual, rapid, and highly specific detection method that can be used as a potential identification tool for Congo Basin and West African mpox.
5 CONCLUSION
In this study, a novel detection protocol that integrates RPA preamplification and CRISPR/Cas12a-mediated trans-cleavage of the ssDNA reporter was established to accurately identify Congo Basin and West African mpox. In the CRISPR-RPA system, the trans-cleavage of ssDNA probe has been reported using real-time fluorescence readout and visualization detection (UV light and imaging system). The CRISPR/RPA assay established in the current study demonstrated excellent capabilities for identifying target plasmids and simulated clinical samples. In summary, the developed CRISPR/RPA assay is a visual, rapid, sensitive, and highly specific detection technique that can be used as an attractive potential identification tool for Congo Basin and West African mpox in resource-limited laboratories.
AUTHORS CONTRIBUTION
Xinggui Yang and Shijun Li conceived and designed the study. Shijun Li and Yi Wang supervised this study. Xinggui Yang, Xiaoyan Zeng, Xu Chen, Junfei Huang, Xiaoyu Wei, Xia Ying, Qinqin Tan, Qinqin Tan, and Shijun Li conducted the experiments. Xinggui Yang, Xia Ying, Shijun Li, Yi Wang, and Xiaoyan Zeng analyzed the data. Shijun Li contributed the reagents and analytical tools. Shijun Li, Shijun Li, Shijun Li, and Yi Wang prepared the materials. Xin Y developed a software program for this study. Xinggui Yang and Xiaoyan Zeng drafted the manuscript. Shijun Li and Yi Wang revised the manuscript.
ACKNOWLEDGMENTS
This study was funded by the Science and Technology Department of Guizhou Province ([2016]4021 and [2018]5606).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Human Ethics Committee of the Guizhou Provincial Center for Disease Control and Prevention (Approval No. Q2023-01) and the Second Affiliated Hospital of the Guizhou University of Traditional Chinese Medicine (Approval No. KYW2022009), and complied with the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
All data sets generated for this research are contained in the manuscript and Supporting Information.




