HTLV-1 persistent infection and ATLL oncogenesis
Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is an oncogenic retrovirus; whereas HTLV-1 mainly persists in the infected host cell as a provirus, it also causes a malignancy called adult T-cell leukemia/lymphoma (ATLL) in about 5% of infection. HTLV-1 replication is in most cases silent in vivo and viral de novo infection rarely occurs; HTLV-1 rather relies on clonal proliferation of infected T cells for viral propagation as it multiplies the number of the provirus copies. It is mechanistically elusive how leukemic clones emerge during the course of HTLV-1 infection in vivo and eventually cause the onset of ATLL. This review summarizes our current understanding of HTLV-1 persistence and oncogenesis, with the incorporation of recent cutting-edge discoveries obtained by high-throughput sequencing.
1 INTRODUCTION
Human T-cell leukemia virus type 1 (HTLV-1) was discovered in the 1980s, but it is estimated to have been infecting humans for more than ten thousand years.1 With over 90% of its infection being asymptomatic, HTLV-1 can spread quietly among hosts, thereby persisting in humans for long time. Five percent of HTLV-1 infection eventually turns lethal due to the development of ATLL, indicating persistent HTLV-1 infection in vivo has a risk to become oncogenic. In addition, HTLV-1 is also the etiological agent of several inflammatory diseases including HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). To better understand HTLV-1 persistence and oncogenesis, essential concepts of HTLV-1 infection are revisited and also updated to incorporate novel insights acquired recently by high-throughput methodologies.
2 HTLV-1 CELLULAR TROPISM
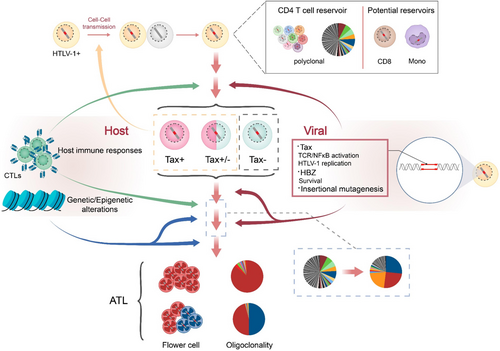
The first step to establishing a successful infection is the viral entry of its target cell. HTLV-1 entry depends on specific receptors including glucose transport 1 (GLUT1),2 heparan sulfate proteoglycans (HSPG),3 and neuropilin 1 (NRP1)4; these are ubiquitously expressed surface proteins5 so that it explains why many cell types are susceptible to HTLV-1 infection in vitro. On the contrary, HTLV-1 is detected predominantly in CD4 T cells in vivo, and to a much lesser extent in CD8 T cells, B cells, and other types of cells (Figure 1). Interestingly, HTLV-1 likely resides in hematopoietic stem cells (HSC) as well, and can persist as HSC develops into T cell.8 On the other hand, HTLV-1 also directly infects monocytes, which may constitute an important viral reservoir in vivo.9, 10 Nevertheless, the CD4 T-cell tropism of HTLV-1 is unlikely a resultant of selective receptor preference, but may rather be determined by postinfection events such as the long-term persistence of infected CD4 T cells (Figure 1). In support of this assumption, the clonal expanding capability of T cells indeed allows them to rapidly proliferate after HTLV-1 infection, thereby comprising a more abundant viral reservoir. However, it is unclear why CD4 T cell seems more permissive to HTLV-1 infection in vivo than CD8 T cell or B cell that can also undergo clonal proliferation. Host factors that support or restrict HTLV-1 replication but are expressed distinctly in different types of blood cells might be another determinant of its tropism in vivo. For instance, HTLV-1 antagonists TCF1 and LEF1 are abundantly expressed in thymocytes but not peripheral T cells, rendering the latter more permissive to HTLV-1.11
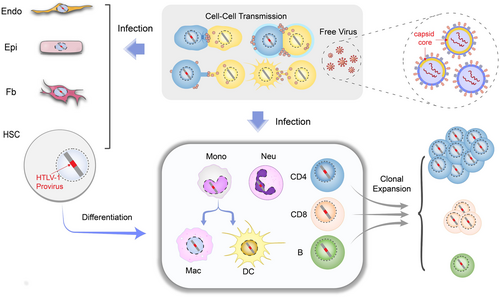
3 HTLV-1 DE NOVO INFECTION
HTLV-1 depends mostly on cell–cell contact to establish de novo infection or cellular transmission, due to its poor infectivity as a free virus. It is estimated that HTLV-1 infection is ten thousand-fold less efficient via free virus than by cell–cell contact.12 The underlying mechanism is a puzzle but recent observations by cryo-electron tomography (EM) offer a possible explanation.13, 14 Cryo-EM performed on an HTLV-1 infected cell line MT-2 (RRID:CVCL_2631), a regularly used donor cell line for viral cell–cell infection, suggests HTLV-1 virions are polymorphic and most HTLV-1 virions observed do not have a complete capsid core13 (Figure 1); this would dampen the infectivity of HTLV-1 free virus. HTLV-1 cell-to-cell transmission is manifested in different fashion,6, 15 including a specialized structure called the virological synapse,16 a transient membrane extension termed cellular conduit,17 extracellular viral assembly18 and dendritic cell-mediated spreading.19 However, which of the aforementioned manner dominates in vivo requires further investigation.
4 HTLV-1 PROVIRUS
HTLV-1 reverse transcribes its RNA genome into DNA and integrates it into chromosome after entry, thereby persisting as an intracellular provirus. Retrovirus requires certain host protein(s) as a cofactor of its integrase for efficient integration, such as the HIV integration cofactor lens epithelium-derived growth factor p75 (LEDGF/p75).20 Phosphatase 2A (PP2A) has been identified as an integration cofactor of HTLV-1,21 which via its B56 domain forms an intasome with HTLV-1 integrase and is thought to play a role in mediating HTLV-1 integration into transcriptionally active regions of the genome.22, 23 High-resolution structural characterization of HTLV-1 intasome is subsequently achieved,22, 23 which improves our understanding of HTLV-1 integration and may help develop novel therapeutic interventions for HTLV-1 infection.
The integrated HTLV-1 provirus has two identical long terminal repeats (LTR) in its 5′ and 3′ terminus, respectively, each acting as a promoter for sense or antisense genes (Figure 2). HTLV-1 structural genes and regulatory/accessory genes including Tax, Rex, p12, p21, p13, and p30 are sense genes transcribed by 5′ LTR whereas regulatory gene HBZ is transcribed by 3′ LTR in the antisense direction (Figure 2). Tax and HBZ are the two viral oncogenes of HTLV-1 that have attracted extensive research interests.24 Tax activates 5′ LTR to boost viral transcription, but its expression is difficult to detect in vivo due to genetic mutation and 5′ LTR inactivation (Figure 2). In contrast, HBZ expression is constantly detected and 3′ LTR also remains constitutively active. Recently a novel HTLV-1 enhancer located close to 3′ LTR that increases HBZ expression was identified,26 the presence of which may further maintain HTLV-1 antisense transcription by antagonizing epigenetic silencing on 3′ LTR.26
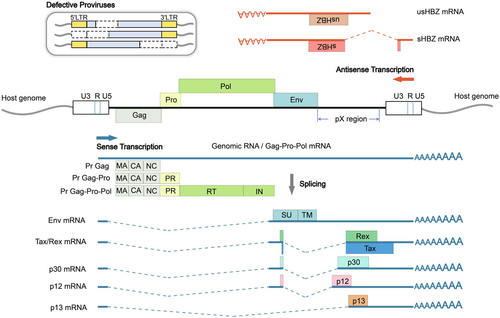
Insertional mutagenesis had rarely been considered as a strategy of HTLV-1 persistence until recently it was discovered that HTLV-1 integration sites tend to appear near transcriptionally active genomic regions,27-29 including those of cancer driver genes.30 These findings suggest a mechanism of persistent HTLV-1 infection via cis-perturbation of host genes by the provirus. This is also supported by another notable finding that identifies a CCCTC-binding factor (CTCF)-binding site in HTLV-1 provirus; CTCF is a ubiquitously expressed transcription factor with important insulator activity31 and the CTCF-binding site thus may create a loop between HTLV-1 sequence and chromosome via CTCF dimerization32, 33 and proposes a possibility of HTLV-1 regulating the three-dimensional structure of chromatin for gene dysregulation and viral persistence.34
5 TAX AND HBZ
Tax and HBZ are currently acknowledged as two HTLV-1 oncogenes with quite distinct mechanisms for oncogenesis. Tax is able to activate important cellular pathways including those responsible for T-cell activation and expansion,35 and is thereby considered a key player in HTLV-1 persistence. Meanwhile, Tax appears to be a strong antigen that triggers cytotoxic T cell (CTL) response killing HTLV-1 infected cells effectively. Therefore, the presence of Tax seems to be a double-edged sword as it is required for viral replication and activation of infected T cells on the one hand, it also exposes HTLV-1 to immune clearance. Under the selective pressure from the host immune system, HTLV-1 likely adapts in vivo to repress or lose Tax expression, which is supported by the fact that Tax is nondetectable in freshly isolated HTLV-1 infected cells but could be detected after ex vivo culture.36, 37 Furthermore, near half of ATLL patients have no Tax expression38 and infected cells without Tax may even proliferate faster in vivo.25 Latest single-cell analyses suggest an unprecedented intermittent expression pattern of Tax that is stress-inducible and is usually detected as a transcriptional burst,39, 40 which represents a subtle strategy of Tax promoting the persistence of the infected cell while evading immune surveillance.41 In contrast, tax expression seems to be more persistent in HAM/TSP than in ATLL and may even predict disease progression in the former,42, 43 suggesting distinct mechanisms of pathogenesis are responsible for the development of these two diseases.44, 45
HBZ is the only viral gene constitutively expressed throughout HTLV-1 infection46 and its expression clearly demarcates infected from uninfected cells.47 Interestingly, HBZ exhibits activities generally opposite to Tax in various aspects48; many cellular pathways including nuclear factor-κB (NFκB) that could be activated by Tax are suppressed by HBZ.24 This might reflect a need of fine-tuning host cell functions to facilitate viral infection, indeed, despite suppressing Tax-mediated viral replication in vitro,49 HBZ somehow appears to enhance HTLV-1 infectivity in vivo.50 A key role of HBZ is to maintain HTLV-1 persistence by promoting the survival of the infected cell, as demonstrated by both HBZ overexpression and depletion assays.24 CTLs recognizing HBZ can effectively kill HTLV-1 infected cells,51 further coupling the presence of HBZ to HTLV-1 persistence. More importantly, HBZ is much less immunogenic than Tax, which contributes to immune evasion by HTLV-1 in vivo. Another unique feature of HBZ as an oncogene is the bifunctional nature of its messenger RNA (mRNA), which can further act as a long noncoding RNA that represses HTLV-1 replication52 and augments the growth of the infected cell.11, 46, 53 With a predominantly nuclear localization of its mRNA,11, 40 HBZ can further curtail host anti-HTLV-1 immune response by limiting its protein expression.
6 HTLV-1 CLONALITY
Advances in high-throughput sequencing allow quantification of the relative abundance of HTLV-1 infected clones by calculating the presence of HTLV-1 integration sites in the host. Every HTLV-1 infected primary cell contains one copy of HTLV-1 provirus in most cases54 and HTLV-1 infected individuals have been estimated to harbor 103 to 106 different HTLV-1 clones.55 It should be noted that HTLV-1 clonality does not seem to correlate in any way with proviral load (PVL),27 which normally increases as HTLV-1 infection progressing from asymptomatic to oncogenic. The clonality of HTLV-1 undergoes dynamic change during the course of infection, with clones emerging and disappearing. In the case of ATLL, a dominant clone, in some cases more than one, emerges and eventually causes the malignancy.54, 56 Longitudinal study discovers that HTLV-1 infected asymptomatic carriers (AC) exhibiting oligoclonality or monoclonality with largely expanded clones progressed to ATLL subsequently, suggesting a high risk of oncogenesis in HTLV-1 infection with an ATLL-like clonality.56, 57 Therefore, HTLV-1 clonality may serve as a valuable biomarker to predict HTLV-1 malignant transformation. Interestingly, a dominant HTLV-1 clone may not always become leukemic, instead it could be outgrown by an emerging clone that evolves to be malignant at last.58
7 GENETIC AND EPIGENETIC ABERRATIONS IN HTLV-1 TRANSFORMED CELLS
Cancer development including ATLL requires genetic and epigenetic alterations.59 Comprehensive genomic studies reveal that many cancer driver genes are mutated in ATLL patients, including the well-known tumor suppressor TP53 (HGNC:11998).60, 61 Notably, ATLL patients accumulate mutations significantly in T-cell receptor (TCR)/NFκB related genes,60, 62 many of which are found to be overlapped with Tax interactome; this explains at least in part the constitutive NFκB activation in Tax-negative ATLL, and also emphasizes its crucial importance for HTLV-1 persistence and oncogenesis. Immunosurveillance is another pathway profoundly affected in ATLL by genetic mutations, which include genes involved in antigen recognition such as HLA-A (HGNC:4931), HLA-B (HGNC:4932) and CD58 (HGNC:1688).60, 61 ATLL cases with the above genes mutated have a worse prognosis, agreeing with the critical role of evading immunosurveillance in ATLL development.63 A recent study reports downregulated expression of CD48 in ATLL, which helps ATLL cells evade NK cell-mediated cytotoxicity.64
ATLL cells exhibit prominent DNA hypermethylation of promoter-associated CpG islands, which causes a number of genes transcriptionally silenced.60 In particular, MHC-I genes were silenced due to CpG hypermethylation in many ATLL cases,60 which is assumed to hinder viral recognition and contribute to immune evasion. On the other hand, histone modifications especially H3K27me3 accumulation is frequently detected in ATLL cells, resulting in the silencing of a panel of genes including several tumor suppressor genes.65 Polycomb-repressive complex 2 (PRC2) is responsible for the global H3K27me3 modification in ATLL and one of its components EZH2 is key to this activity,65 pharmacologic inhibition which reverses epigenetic disruption selectively eliminates leukemic cells.66 Intriguingly, both Tax and HBZ could interact with core components of PRC2 and induce H3K27me3 accumulation,66, 67 indicating they may play important roles in the global epigenetic reprogramming of HTLV-1 infected cells.
8 TARGETED THERAPEUTICS FOR ATLL
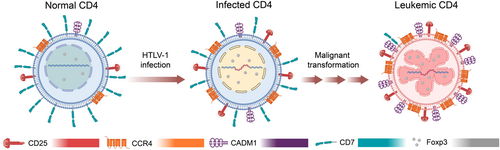
ATLL cell has a mature T-cell phenotype with the expression of CD25 and CC chemokine receptor 4 (CCR4) but lacking CD7, or sometimes appears like regulatory T cell (Treg) due to forkhead box P3 (Foxp3) expression (Figure 3). It is likely that the expression of these surface markers is a result of HTLV-1 infection, but a possibility of selective infection in mature T cell or other cell types by HTLV-1 could not be completely ruled out. Cell adhesion molecule 1 (CADM1) is a recently discovered surface marker that is widely upregulated during HTLV-1 infection and can reflect disease progression along with CD7, with CADM1 + CD7-indicating a premalignant state of infected cells.69 Nevertheless, ATLL is extremely aggressive and most patients suffer from a poor prognosis. Standard treatments of ATLL mainly include intensive chemotherapy and a zidovudine/interferon α (AZT/IFN-α) combination therapy; however, these treatments are not satisfactory, particularly in aggressive types of ATLL, and targeted therapy has been explored with high hope. Many cellular genes that are dysregulated by HTLV-1 have been reported and might serve as possible ATLL targets (Figure 4), including bromodomain-and-extra-terminal-domain (BET) proteins70, 71 and oxysterol-binding protein-related protein 4L (ORP4L)72 identified very recently, yet solid translational work is required to evaluate their druggability and effectiveness for targeted ATLL therapy. In addition, epigenetic inhibitors are an alternative option for ATLL treatment, and targeting EZH2 and HDAC has yielded promising results in preclinical and phase I trials.73
Various humanized antibodies have been developed for immunotherapy and examined for their efficacy in treating ATLL, however, only the CCR4-antibody mogamulizumab is clinically approved for treating relapsed or refractory ATLL.74 A Tax peptide-pulsed dendritic cell (Tax-DC) vaccine that augments Tax-specific CTL response demonstrated efficacy in a clinical trial,75 which may be useful for ATLL patients with Tax expression. HBZ is presumably a more effective target for its constant expression in ATLL and its requirement for ATLL cell survival. However, the low immunogenicity of HBZ protein poses a major obstacle to traditional vaccine development despite that HBZ vaccination generates protective CTLs in vivo.76 Alternatively, inhibiting HBZ RNA by oligonucleotide therapeutics might be a more practical idea.
9 CONCLUDING REMARKS
Multiple hits are required for cancer onset77 and so is HTLV-1-mediated oncogenesis. A proposed model for HTLV-1 persistence and oncogenesis is presented in Figure 5. It is presumed that HTLV-1 provides the first hit, either through actions of viral oncogenes Tax and HBZ or by potential insertional mutagenesis. Genetic/epigenetic aberrations accumulated in the host cell, particularly those causing cancer-driver gene mutations, provide the rest hits and promote leukemic transformation. The transition of HTLV-1 from persistence to oncogenesis involves excessive T-cell activation78, 79 and the acquisition of a Treg signature in infected cells.47, 79 Immune evasion is required for the persistence of both nonmalignant and malignant HTLV-1 infected cells, and HTLV-1 clones with selective advantage in proliferation and immune evasion are most likely to cause ATLL eventually.62
AUTHOR CONTRIBUTIONS
Xiaorui Zuo and Guangyong Ma wrote the review. Xiaorui Zuo, Ruoning Zhou, Sikai Yang, and Guangyong Ma were involved in discussions and critical reading of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (32070155 and 32270171 to G. M.) and China Pharmaceutical University (3154070031 and 3342200014 to G. M.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We apologize that some primary or historical publications might have not been cited due to space limitations.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




