Clinical and laboratory features and factors predicting disease severity in pediatric patients with hemorrhagic fever with renal syndrome caused by Hantaan virus
Abstract
The clinical features and factors associated with disease severity in children with hemorrhagic fever with renal syndrome (HFRS) have not been well characterized. This study analyzed the clinical and laboratory factors associated with disease severity in children with HFRS caused by Hantaan virus. Data in pediatric patients with HFRS were retrospectively collected from Xi'an Children's Hospital over a 9-year period. Independent factors associated with disease severity were identified. Nomogram predicting disease severity was constructed based on variables filtered by feature selection. In total, 206 children with HFRS were studied. Fever, digestive tract symptoms, headache, backache, bleeding, and renal injury signs were the common symptoms. Elevated white blood cell, reduced platelet, hematuria, proteinuria, coagulation abnormalities, increased blood urea nitrogen (BUN) and procalcitonin (PCT), decreased estimated glomerular filtration rate and low serum Na+, Cl−, and Ca2+ were the common laboratory findings. In the 206 patients, 21 patients had critical type disease and 4 patients (1.9%) died. Hydrothorax, hypotension and cerebral edema/cerebral herniation at hospital admission were independent clinical characteristics, and neutrophil %, prothrombin activity, PCT, BUN, and Ca2+ at hospital admission were independent laboratory factors associated with critical disease. Feature selection identified BUN, PCT and prothrombin time as independent factors related to critical disease. A nomogram integrating BUN and PCT at admission was constructed and calibration showed high accuracy for the probability prediction of critical disease. In conclusion, this study characterized the clinical and laboratory features and constructed a nomogram predicting disease severity in pediatric HFRS, providing references for disease severity evaluation in managing children HFRS.
1 INTRODUCTION
The genus orthohantavirus in Hantaviridae family under Bunyavirales Order includes emerging pathogens worldwide that cause two human syndromes, hantavirus cardiopulmonary syndrome (HCPS) in the Americas and hemorrhagic fever with renal syndrome (HFRS) in Eurasia.1-4 There are mainly four orthahantaviruses, namely Hantaan, Seoul, Puumala, and Dobrava-Belgrade viruses, that have been linked to human HFRS.5-8 Infection of humans by the different orthahantaviruses manifests different clinical course and disease severity and these viruses are endemic at different geographic areas. Hantaan virus and Seoul virus, the two major pathogens of HFRS in Asia including China and Korea, induce a severe form and a less severe form of HFRS, respectively.7-10 Puumala virus and Dobrava-Belgrade virus, the two major etiologies of HFRS in Europe, are associated with a milder form of HFRS known as nephropathia epidemica (NE) and a severe form of HFRS, respectively.11-15
The pathogenesis of HFRS is characterized by enhanced capillary vascular permeability, endothelial dysfunction, and blood coagulation alteration.16 The typical and common manifestations of HFRS include fever, hemorrhage, and acute kidney injury. The disease course of clinically typical HFRS may include five distinctive but sometimes overlapping phases including febrile, hypotensive, oliguric, diuretic, and convalescent phases.17 In China, HFRS remains a serious public health issue. China has about 90% of the total HFRS cases worldwide, with approximately 20 000–50 000 patients reported yearly18, 19 and a mortality rate of 0.1%–15%.20
Since most cases of HFRS are reported in adults, and children are in general believed to be rarely affected by orthahantaviruses,21-26 the clinical and laboratory characteristics of HFRS in children have been only investigated in small number of patients or case reports. In NE, the mild form HFRS, some studies showed that the clinical course was similar in adults and children with high frequency of acute kidney injury (AKI) and thrombocytopenia, and with no severe complications.27, 28 However, there are other studies indicating that the clinical picture of NE seems to be less severe in children than in adults.23, 29-31 In the infection of highly pathogenic orthahantaviruses, such as Sochi virus, a new genotype of the Dobrava-Belgrade virus (DOBV) species,12 studies showed that the frequency of clinical cases and their clinical course are comparable between children and adults.32 A retrospective review of 63 cases of HFRS in children from Korea showed that the clinical and laboratory findings were in general similar between children and adults.21 However, there are atypical cases of HFRS in children without distinctive manifestations and typical disease progresses.33 A retrospective analysis on the clinical data in 26 children with HFRS from China showed that the clinical manifestations of pediatric HFRS may be atypical and should be distinguished from other febrile disease such as cold or appendicitis at the early stage.34 Overall, the clinical picture of HFRS among children is not well characterized and factors associated with the disease severity and prognosis have not been well identified. Moreover, the classification of disease severity in HFRS is performed based on the clinical profiles of the whole disease course and no approach is currently available for the prediction of disease severity and prognosis in pediatric HFRS patients.
Therefore, we carried out this study in a relatively large cohort of pediatric patients from a large children's hospital in northwest China. In this endemic area, Hantaan virus is the etiology of HFRS.35-37 We aimed to characterize the clinical and laboratory features of pediatric HFRS, identify factors associated with the disease severity/prognosis and construct a nomogram predicting the disease severity using laboratory data collected at the day of hospital admission.
2 METHODS
2.1 Study populations
This study was a retrospective study. The study population included patients diagnosed HFRS in the Xi'an Children's Hospital, the largest children's hospital in northwest China, during a 9-year period from January 2012 to December 2020. In addition to the clinical manifestation and laboratory findings, the diagnosis of HFRS in all the patients was confirmed by the positive results of specific IgM against Hantaan virus.38 The inclusion criteria were (1) age <18-year-old; and (2) diagnosed as HFRS according to the diagnostic criteria of the Prevention and Treatment Proposal of HFRS by the Ministry of Health, People's Republic of China.38 The exclusion criteria included (1) patients with pre-existing diseases (including diseases of the heart, liver, kidney, nervous system, blood, and other systems); and (2) patients with incomplete data. The study was performed in accordance with the Declaration of Helsinki. The use of the data and the research protocol were approved by the Ethics Committee of Xi'an Children's Hospital (No. 20210035). The requirement of informed consent from the study subjects or their guardians was waived because of the de-identification of information in the subjects before inclusion in the analysis and the retrospective nature of the study, with the approval of the Ethics Committee of Xi'an Children's Hospital.
2.2 Data collection
Demographic, clinical, and laboratory data at hospital admission in the patients were collected. Demographic data included gender, age, weight, residential area (urban or rural), and feeding pattern in infancy. Clinical data included the hospital stay, morbidity season, day of illness at admission, symptoms (stomachache, fever, headache, nausea and vomiting, backache, bleeding, etc.), hydropericardium, hydrothorax, ascites, oliguria, polyuria, proteinuria, hypotension, cerebral edema/cerebral herniation, pneumonia, ventilator therapy and continuous renal replacement therapy (CRRT). Laboratory data included hematology, urine routine test, coagulation test, liver and renal biochemistry, creatine kinase MB isoenzyme (CK-MB), and electrolytes (K+, Na+, Cl−, and Ca2+) at hospital admission. Hypotension was defined as a systolic blood pressure less than the 5th percentile of normal for age, namely: <70 mm Hg + (2 × age in years) in children 1 to 10 years and <90 mm Hg in children ≥10 years of age.39 The diagnosis of pneumonia was mainly based on clinical symptoms such as fever, cough, respiratory distress, and moist rales and imaging examination showing acute pulmonary infiltrate/consolidation.40 The image findings including X-ray/CT-scan of the abdomen and thorax that was performed based on the discretion of the physician in some patients were also included. The disease severity in the patients was classified into mild, moderate, severe and critical types performed at the day of patient discharge or the day of death in the died patients primarily based on the criteria described previously (Supporting Information: Table S1).38, 41
2.3 Outcome measure
Considering the small number of death cases and the fact that all the died patients had critical type disease, we used the critical type disease in stead of the mortality in the patients as outcome measure of the study.
2.4 Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median. The t-test and Mann-Whitney U-test were used to compare the two sets of data. Categorical variables were expressed by frequency (percentage). Pearson's χ2 or Fisher's exact tests were used for data comparison. Predictive factors associated with disease severity (critical disease) were established by multivariate logistic regression. The receiver operating characteristic (ROC) curve was used to calculate the sensitivity and specificity of the factors related to critical disease and the maximizing Youden index. The area under the curve (AUC) and the sensitivity and specificity were used to evaluate the accuracy of the factors for predicting the outcome. Feature selection was used to filter suitable variables via the information gain method. Factors with selected variable attributes >0.1 were used for logistic regression. Nomogram by integrating the independent factors of logistic regressions was established to visualize the outcome probabilities in the predictive model. Calibration plots were used to evaluate the accuracy of the nomogram, and the calibration curve was used to represent the correlation between actual results and predicted probabilities. Hosmer-Lemeshow was used to evaluate the goodness of fit of the model. SPSS (version 25.0), Orange (Version 3.24.1), and Medcalc statistical software (version 18.1) were used for data analyses. A p < 0.05 was regarded as statistically significant.
3 RESULTS
3.1 Characteristics of the study population
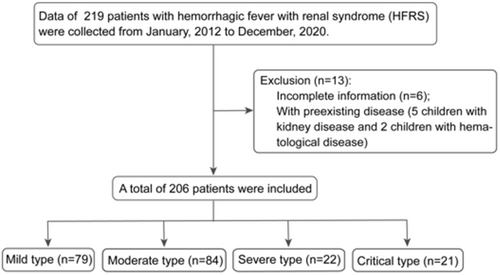
Data of 219 pediatric patients diagnosed HFRS were collected during the study period. Seven patients with pre-existing diseases (five with kidney disease and two with hematological diseases) and six patients with incomplete data were excluded. Finally, 206 patients were included in the study. All the included patients met the inclusion criteria. The clinical types of the disease in the 206 patients included 79 mild, 84 moderate, 22 severe and 21 critical types (Figure 1) referred to the diagnostic criteria (Supporting Information: Table S1).38, 41
The characteristics of all the pediatric patients and patients with different clinical types of HFRS are presented in Supporting Information: Table S2. Males (66%) accounted for a larger proportion of the patients. The average age of the patients was 8.2 ± 3.4 years with ages between 5 and 12 years accounting for most of the patients, and the morbidity seasons were mainly in autumn and winter (Supporting Information: Figure S1). A larger proportion of patients were rural residents (83%) and the feeding pattern in infancy was mainly breast feeding. Almost all of the patients had fever (98.1%). Most of the patients had digestive tract symptoms including stomachache (63.1%) and nausea/vomiting (62.1%). Headache and backache presented in 45.1% and 66% of the patients, respectively. Bleeding symptoms including petechia and/or ecchymosis in skin or oral mucosa, conjunctival hemorrhage, hematuria, and pulmonary hemorrhage were found in 47.1% of the patients. Hydropericardium was recorded in severe (9.1%) and critical (23.8%) types of patients. Hydrothorax was also mainly presented in severe (31.8%) and critical (76.2%) types of patients. Ascites presented in 40.9% of severe and 61.9% of critical type patients, respectively. Nearly half of the severe (45.5%) and 85.7% of the critical type patients had oliguria. Polyuria was recorded in 63.6% and 47.6% of the severe and critical type patients, respectively. Proteinuria was presented in almost all the patients (93.7%). Hypotension was mainly found in severe (27.3%) and critical (52.4%) type patients. Cerebral edema/herniation and pneumonia were primarily found in patients with critical type disease (47.6% and 38.1%, respectively). Ventilator was needed in 33.3% of the critical type patients. In all the patients, 11.7% of them needed CRRT (27.3% of severe type and 85.7% of critical type of the patients, respectively, Supporting Information: Table S2).
The laboratory parameters of all the patients and patients with different clinical types of HFRS at hospital admission are presented in Supporting Information: Table S3. At hospital admission, most children had elevated white blood cell (WBC) and reduced platelet (PLT). Hemoglobin (HB) was elevated in moderate, severe and critical types of the patients. Hematuria and proteinuria were common in all the patients, especially in the severe and critical types of patients. Coagulation abnormalities were also common in the patients. Procalcitonin (PCT) was significantly elevated in severe and critical types of the patients. The estimated glomerular filtration rate (eGFR) was sequentially and significantly decreased from mild, moderate, severe to critical types of the patients. Creatinine (Cr) and uric acid (UA) were sequentially elevated from mild, moderate, severe to critical types of the patients. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were elevated and albumin (ALB) levels were decreased sequentially from mild, moderate, severe to critical types of the patients. CK-MB was elevated in severe and critical types of the patients. Low Na+ was presented in moderate, severe and critical types of patients and low Cl− and Ca2+ were presented in severe and critical types of patients (Supporting Information: Table S3).
Among the 206 patients, 26 patients (12.6%) were admitted to intensive care unit (ICU) for management. Of these 26 patients, 21 patients (10.2%) meet the diagnostic criteria of critical type disease and 5 patients who had much severe disease condition but did not meet the diagnostic criteria of critical type were admitted to ICU according to the discretion of physician. Four patients died, with a mortality rate of 1.9%. All of the four died patients had critical type disease and required management in ICU. Characteristics of the four died pediatric HFRS patients were shown in Supporting Information: Table S4.
3.2 Factors associated with critical disease
The patients were categorized into noncritical group and critical group and factors associated with critical disease were analyzed.
The demographic, epidemiological, and clinical characteristics of the pediatric HFRS patients with noncritical and critical disease were shown in Table 1. Univariate and then multivariate logistic regression showed that hydrothorax (OR 18.36, 95% CI 3.25, 103.64, p < 0.001), hypotension (OR 12.83, 95% CI 2.06, 79.96, p = 0.006), and cerebral edema/cerebral herniation (OR 16.88, 95% CI 2.44, 116.83, p = 0.004) were independent risk factors for critical disease (Table 2).
| Noncritical (n = 185) | Critical (n = 21)a | p Value | |
|---|---|---|---|
| Male, n (%) | 122 (65.9%) | 14 (66.7%) | 0.947 |
| Age, years [mean (range)] | 8.04 ± 3.32 (1.17–15) | 9.67 ± 3.55 9.67 (2.58–16) | 0.034 |
| Residential area | 0.214 | ||
| Urban, n (%) | 34 (18.4%) | 1 (4.8%) | |
| Rural, n (%) | 151 (81.6%) | 20 (95.2%) | |
| Hospital stay, days [mean (range)] | 8.14 ± 3.39 (2-32) | 16.95 ± 9.89 (1–36) | 0.001 |
| Morbidity season | 0.658 | ||
| Spring, n (%) | 15 (8.1%) | 2(9.5%) | |
| Summer, n (%) | 12 (6.5%) | 2 (9.5%) | |
| Autumn, n (%) | 83 (44.9%) | 11 (52.4%) | |
| Winter, n (%) | 75 (40.5%) | 6 (28.6%) | |
| Duration of illness at admission, days [mean (range)] | 4.96 ± 1.81 (1–10) | 4.67 ± 1.20 (3–7) | 0.330 |
| Body weight, kg | 27 (20,37) | 36 (27,42.25) | 0.019 |
| Maximum body temperature, °C [mean (range)] | 39.49 ± 0.65 (37.50–42.00) | 39.65 ± 0.51 (38.5–40.7) | 0.282 |
| Feeding pattern in infancy | 0.025 | ||
| Breast feeding, n (%) | 108 (58.4%) | 18(85.7%) | |
| Formula feeding, n (%) | 43 (23.2%) | 1(4.8%) | |
| Mixed feeding, n (%) | 34 (18.4%) | 2(9.5%) | |
| Symptom | |||
| Stomachache, n (%) | 111 (60%) | 19 (90.5%) | 0.006 |
| Fever, n (%) | 181 (97.8%) | 21 (100%) | 1.000 |
| Headache, n (%) | 79 (42.7%) | 14 (66.7%) | 0.037 |
| Nausea/vomiting, n (%) | 111 (60%) | 17 (81%) | 0.061 |
| Back pain, n (%) | 119 (64.3%) | 17 (81%) | 0.127 |
| Bleeding, n (%) | 81 (43.8%) | 16 (76.2%) | 0.005 |
| Hydropericardium, n (%) | 4 (2.2%) | 5 (23.8%) | 0.001 |
| Hydrothorax, n (%) | 15 (8.1%) | 16 (76.2%) | <0.001 |
| Ascites, n (%) | 43 (23.2%) | 13 (61.9%) | <0.001 |
| Oliguria, n (%) | 37 (20%) | 18 (85.7%) | <0.001 |
| Polyuria, n (%) | 60 (32.4%) | 10 (47.6%) | 0.164 |
| Proteinuria, n (%) | 172 (93%) | 21 (100%) | 0.370 |
| Hypotensionb, n (%) | 14 (7.6%) | 11 (52.4%) | <0.001 |
| Cerebral edema/cerebral herniation, n (%) | 5 (2.7%) | 10 (47.6%) | <0.001 |
| Pneumoniac, n (%) | 15 (8.1%) | 8 (38.1%) | 0.001 |
| Ventilator use, n (%) | 0 | 7 (33.3%) | <0.001 |
| CRRT, n (%) | 6 (3.2%) | 18 (85.7%) | <0.001 |
- Abbreviations: CRRT, continuous renal replacement therapy; HFRS, hemorrhagic fever with renal syndrome.
- a All the four died patients had critical type disease and were included in this group of patients.
- b Hypotension is a systolic blood pressure less than the 5th percentile of normal for age, namely: <70 mmHg + (2 × age in years) in children 1 to 10 years and <90 mmHg in children ≥10 years of age.39
- c The diagnosis of pneumonia is mainly based on clinical symptoms such as fever, cough, respiratory distress, and moist rales and imaging examination showing acute pulmonary infiltrate/consolidation.40
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age, years | 1.17 (1.01,1.35) | 0.038 | ||
| Body weight, kg | 1.04 (1.01,1.07) | 0.018 | ||
| Stomachache | 6.33 (1.43,28.00) | 0.015 | ||
| Headache | 2.68 (1.03,6.96) | 0.042 | ||
| Bleeding | 4.11 (1.44,11.69) | 0.008 | ||
| Hydropericardium | 14.14 (3.45,57.96) | <0.001 | ||
| Hydrothorax | 36.27 (11.66,112.78) | <0.001 | 18.36 (3.25,103.64) | 0.001 |
| Ascites | 5.37 (2.09,13.80) | <0.001 | ||
| Oliguria | 24.00 (6.71,85.82) | <0.001 | ||
| Hypotension | 13.44 (4.87,37.07) | <0.001 | 12.83 (2.06,79.96) | 0.006 |
| Cerebral edema and/or cerebral herniation | 32.73 (9.53,112.43) | <0.001 | 16.88 (2.44,116.83) | 0.004 |
| Pneumonia | 6.97 (2.50,19.47) | <0.001 | ||
- Abbreviations: CI, confidence interval; HFRS, hemorrhagic fever with renal syndrome; OR, odds ratio.
Laboratory characteristics of the pediatric HFRS patients with noncritical and critical disease were shown in Table 3. Univariate and multivariate logistic regression analysis showed that neutrophil (NEU)% (OR 1.08, 95% CI 1.02, 1.14, p = 0.005), prothrombin activity (PTA) (OR 0.94, 95% CI 0.91, 0.97, p = 0.001), PCT (OR 1.05, 95% CI 1.00, 1.10, p = 0.037), BUN (OR 1.12, 95% CI 1.01, 1.24, p = 0.039), and Ca2+ (OR 0.01, 95% CI 0.00, 0.48, p = 0.022) were independent risk factors for critical disease (Table 4).
| Reference value | HFRS patients | p Valueb | ||
|---|---|---|---|---|
| Noncritical (n = 185) | Critical (n = 21)a | |||
| WBC, × 109/L | 4.0–12.0 | 11.13 (7.79,17.21) | 25.52 (9.34,42.59) | 0.005 |
| NEU, % | 35–70 | 43.06 ± 16.98 | 61.10 ± 11.98 | <0.001 |
| LYM, % | 25–60 | 42 (29.75,54.60) | 28.80 (19.70,34.05) | <0.001 |
| EO, % | 0–9 | 0.50 (0.10,1.20) | 0.40 (0.15,1.00) | 0.693 |
| RBC, × 1012/L | 4.0–5.5 | 4.51 (4.245,5.00) | 4.69 (4.26,5.23) | 0.190 |
| HB, g/L | 110–150 | 128.02 ± 17.48 | 137.62 ± 24.29 | 0.092 |
| PLT, × 109/L | 150–400 | 88 (63,148.50) | 46 (28,61) | <0.001 |
| Hematuria (+) | - | 68 (36.8%) | 15 (71.4%) | 0.002 |
| Proteinuria | - | 0.001 | ||
| + | 48 (25.9%) | 0 | ||
| ++ | 52 (28.1%) | 7 (33.3%) | ||
| +++ | 72 (38.9%) | 13 (61.9%) | ||
| ++++ | 0 | 1 (4.8%) | ||
| PT, s | 9.8–12.1 | 13 (12.30,13.73) | 14.90 (13.79,21.05) | <0.001 |
| PTA,% | 70–120 | 100.40 ± 19.66 | 73.41 ± 26.18 | <0.001 |
| INR | 0.8–1.2 | 1.00 (0.94,1.08) | 1.19 (1.08,1.75) | <0.001 |
| APTT, s | 22.7–31.8 | 42.80 (36.75,51.02) | 65.50 (53.24,82.10) | <0.001 |
| FIB, g/L | 1.8–3.5 | 2.68 ± 0.87 | 1.73 ± 0.97 | <0.001 |
| PCT, ng/mL | 0.00–0.05 | 1.47 (0.42,4.11) | 19.84 (4.66,35.45) | <0.001 |
| eGFR, ml/min/1.73 m2) | ≥90 | 59.89 (30.73,85.90) | 31.97 (21.32,65.34) | 0.040 |
| BUN, mmol/L | 2.5–6.5 | 7.48 (4.21,14.59) | 20.18 (16.57,31.02) | <0.001 |
| Cr, μmoL/L | 15.4–90.4 | 72 (40.85,156) | 237 (149,378.70) | <0.001 |
| UA, μmoL/L | 140–390 | 436 (302.90,693.15) | 615 (492.50,784.50) | 0.004 |
| ALT, U/L | 8–40 | 41 (24,66) | 69 (44.128.5) | 0.004 |
| AST, U/L | 10–40 | 71 (35,130) | 187 (110,367.5) | <0.001 |
| ALB, g/L | 35–50 | 34.20 (30.70,39.65) | 27.60 (24.15,32.65) | <0.001 |
| GLB, g/L | 15–38 | 25.20 (21.50,27.80) | 21.70 (19.90,25.85) | 0.017 |
| CK-MB, IU/L | 0–25 | 26 (17.50,38) | 63 (37.5,92) | <0.001 |
| K+, mmol/L | 3.5–5.5 | 3.86 ± 0.47 | 4.54 ± 1.23 | <0.001 |
| Na+, mmol/L | 135–145 | 134.49 ± 5.63 | 127.16 ± 6.26 | <0.001 |
| Cl−, mmol/L | 98–110 | 99.79 ± 5.14 | 94.09 ± 7.05 | <0.001 |
| Ca2+, mmol/L | 2.0–2.8 | 2.11 ± 0.17 | 1.84 ± 0.33 | <0.001 |
- Abbreviations: ALB, albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca2+, calcium ion; Cl−, chloride ion; CK-MB, creatine kinase MB isoenzyme; Cr, creatinine; eGFR, estimated glomerular filtration rate; EO, eosinophil; FIB, fibrinogen; GLB, globulin; HB, hemoglobin; INR, international normalized ratio; K+, potassium ion; LYM, lymphocyte; Na+, sodium ion; NEU, neutrophil; PCT, prothrombin consumption test; PLT, platelet; PT, prothrombin time; PTA, prothrombin activity; RBC, red blood cell; UA, uric acid; WBC, white blood cell.
- a All the four died patients had critical type disease and were included in this group of patients.
- b Difference is between noncritical and critical type patients.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| WBC, × 109/L | 1.08 (1.04,1.12) | <0.001 | ||
| NEU, % | 1.06 (1.03,1.09) | <0.001 | 1.08 (1.02,1.14) | 0.005 |
| LYM, % | 0.94 (0.91,0.98) | 0.001 | ||
| HB, g/L | 1.03 (1.00,1.06) | 0.025 | ||
| PLT, × 109/L | 0.96 (0.94,0.98) | <0.001 | ||
| Hematuria (+) | 4.30 (1.59,11.61) | 0.004 | ||
| PT, s | 1.43 (1.21,1.69) | <0.001 | ||
| PTA, % | 0.95 (0.93,0.97) | <0.001 | 0.94 (0.91,0.97) | 0.001 |
| INR | 14.71 (3.53,61.24) | 0.000 | ||
| FIB, g/L | 0.23 (0.12,0.46) | 0.000 | ||
| PCT, ng/mL | 1.11 (1.07,1.16) | <0.001 | 1.05 (1.00,1.10) | 0.037 |
| BUN, mmol/L | 1.16 (1.10,1.23) | <0.001 | 1.12 (1.01,1.24) | 0.039 |
| Cr, μmoL/L | 1.01 (1.00,1.01) | <0.001 | ||
| UA, μmoL/L | 1.00 (1.00,1.01) | 0.003 | ||
| ALT, IU/L | 1.01 (1.00,1.01) | 0.001 | ||
| AST, IU/L | 1.00 (1.00,1.01) | 0.001 | ||
| GLB, g/L | 0.90 (0.81,0.99) | 0.036 | ||
| CK-MB, IU/L | 1.03 (1.01,1.04) | <0.001 | ||
| K+, mmol/L | 3.96 (1.87,8.35) | <0.001 | ||
| Na+, mmol/L | 0.84 (0.78,0.91) | <0.001 | ||
| Cl−, mmol/L | 0.85 (0.79,0.92) | <0.001 | ||
| Ca2+, mmol/L | 0.01 (0.00,0.06) | <0.001 | 0.01 (0.00,0.48) | 0.022 |
- Abbreviations: ALB, albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca2+, calcium ion; Cl−, chloride ion; CK-MB, creatine kinase MB isoenzyme; Cr, creatinine; eGFR, estimated glomerular filtration rate; EO, eosinophil; FIB, fibrinogen; GLB, globulin; HB, hemoglobin; HFRS, hemorrhagic fever with renal syndrome; INR, international normalized ratio; K+, potassium ion LYM, lymphocyte; Na+, sodium ion; NEU, neutrophil; PCT, procalcitonin; PLT, platelet; PT, prothrombin time; PTA, prothrombin activity; RBC, red blood cell; UA, uric acid; WBC, white blood cell.
3.3 Accuracy of the independent laboratory factors for predicting the outcome
Considering the objectivity, we evaluated the accuracy of the independent laboratory factors for predicting outcome in the patients.
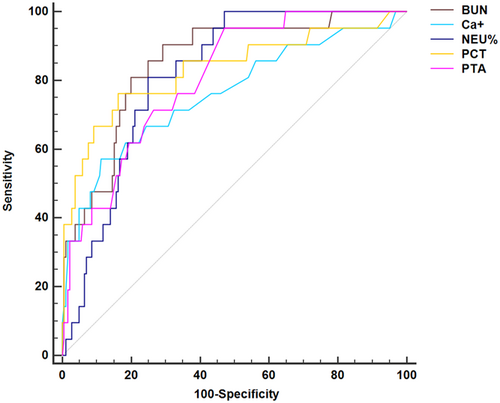
Among the independent risk factors (NEU%, PTA, PCT, BUN, and Ca2+) associated with critical disease, the AUC of NEU% was 0.81 (95% CI 0.74, 0.86), with a sensitivity of 81.0% and a specificity of 72.4%; the AUC of PTA was 0.81 (95% CI 0.75, 0.86), with a sensitivity of 95.2% and a specificity of 53.0%; the AUC of PCT was 0.83 (95% CI 0.77, 0.88), with a sensitivity of 76.2% and a specificity of 83.8%; the AUC of BUN was 0.85 (95% CI 0.80, 0.90), with a sensitivity of 90.5% and a specificity of 70.8% and the AUC of Ca2+ was 0.76 (95% CI 0.69, 0.81), with a sensitivity of 57.10% and a specificity of 88.6% (Table 5, Figure 2).
| AUC | 95% CI | Sensitivity (%) | Specificity (%) | Cut-off value | |
|---|---|---|---|---|---|
| NEU% | 0.81 | 0.74, 0.86 | 81.0 | 72.4 | 50.7 |
| PTA | 0.81 | 0.75, 0.86 | 95.2 | 53.0 | 97.65 |
| PCT | 0.83 | 0.77, 0.88 | 76.2 | 83.8 | 6.20 |
| BUN | 0.85 | 0.80, 0.90 | 90.5 | 70.8 | 13.22 |
| Ca+ | 0.76 | 0.69, 0.81 | 57.10 | 88.6 | 1.89 |
- Abbreviations: AUC, area under the curve; BUN, blood urea nitrogen; Ca+, calcium ion; CI, confidence interval; HFRS, hemorrhagic fever with renal syndrome; NEU, neutrophil; OR, odds ratio; PCT, procalcitonin; PTA, prothrombin activity.
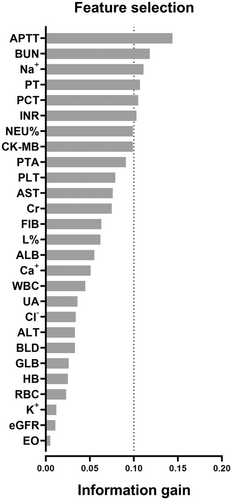
3.4 Feature selection and development of nomogram
Feature selection for laboratory variables associated with critical disease according to the information gain ranking results was shown in Supporting Information: Table S5 and Figure 3. Logistic regression of the top 6 variables (APTT, BUN, Na+, PT, PCT, INR) with information gain >0.1 showed that BUN (OR 1.15, 95% CI 1.07, 1.23, p < 0.001), PCT (OR 1.05, 95% CI 1.00, 1.09, p = 0.035), and PT (OR 1.37, 95% CI 1.09, 1.73, p = 0.007) were independent risk factors for critical disease (Supporting Information: Table S6).
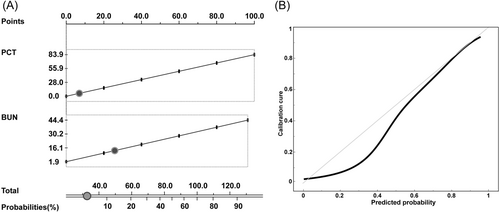
Because BUN and PCT were among the independent risk factors for critical disease in multivariate analysis with higher AUC (0.85 and 0.83, respectively) and were also among the independent risk factors by logistic regression among the top six variables in feature selection, nomogram based on these two parameters was constructed to visualize the probabilities of critical disease (Figure 4A). The points of the calibration plots of the nomogram were generally close to the ideal curve (Figure 4B). The Hosmer–Lemeshow test showed that the degree of fit was not statistically significant (p = 0.355).
4 DISCUSSION
This study, to our knowledge, in a largest pediatric population of HFRS caused by Hantaan virus so far, investigated the clinical features and analyzed factors associated with the disease severity/prognosis. The clinical characteristics and the disease course in the pediatric HFRS patients of this study are mostly similar with those reported in adult patients. Of note, more than 10% of the pediatric patients had critical type disease (10.2%), required management in ICU (12.6%), or needed CRRT (11.7%). Ventilator was also used in 33.3% of the critical type patients. There are some atypical and critical manifestations which may lead to unfavorable outcomes. For instance, cerebral edema/herniation presented in 47.6% of the critical type patients. There is a mortality rate of 1.9% in the pediatric patients of this study. These findings suggest that HFRS caused by Hantaan virus is one of the health threats to children living in the endemic areas.
In the present study, males (66%) accounted for a larger proportion of patients and the patients aged between 5 and 12 years accounted for most of the patients. These results are consistent with previous analyses of children with HFRS.21, 34, 42 Behavioral differences have been indicated to be related to the sex difference in some infectious diseases that men are more frequently affected than females.43 The disease severity between male and female children showed no significant difference in the present study. This is consistent with previous study in NE43 but inconsistent with a study of HFRS patients mostly composed of adults showing that the severity of disease outcome was worse for females.42 In the present study, the age of the children appeared to be older in the children who had more severe disease but it was not independently associated with critical disease. The morbidity seasons of cases in the present study were mainly in autumn and winter and a larger proportion of patients were rural residents (83%). These seasonal and residential features of incidence may be related to the meteorological factors44, 45 and landscape features and rodent community activities in different seasons and regions that may affect the risk of human hantavirus infection.46, 47
Consistent with previous studies,21, 34, 48 the most common symptoms in the children with HFRS were fever, digestive tract symptoms, headache, backache, and hemorrhagic manifestations. Of note, this study revealed that hydrothorax, cerebral edema/cerebral herniation, and hypotension were independently associated with critical disease. Polyserous effusions have been recorded in children with HFRS in a previous study.34 In the present study, serous cavity effusions including hydropericardium, hydrothorax and ascites were primarily presented in severe and critical patients. Although Puumala hantavirus HFRS patients rarely develop symptoms consistent with encephalitis or acute encephalomyelitis,49-51 serious central nervous system (CNS) complications were especially recorded in young males during acute Puumala hantavirus infection.52 Cases of Dobrava hantavirus HFRS encephalitis have also been reported.53 There is case report showing that child with HFRS was misdiagnosed as encephalitis because of fever and headache.33 In the present study, all of the four died children had brain edema and one of them died of brain herniation, indicating that the involvement of CNS is a critical and sometimes a life-threatening sign of children with Hantaan virus HFRS. Development of hypotension is one of the serious clinical manifestations of HFRS. In this study, hypotension is independently associated with critical disease, two of the four died patients had hypotension at hospital admission and 12.1% of the pediatric HFRS patients experienced hypotension, a similar proportion with a report in HFRS children from Korea (11%).21
In this study, 11.7% of the patients (24/206) required CRRT. In a Korean study of HFRS children, 17.5% of the patients (11/63) required dialysis.21 These findings imply that more than 10% of the pediatric patients may have severe acute renal failure that needs renal replacement therapy.
The mortality rate of HFRS varied from 0.1% to 15% in reports mainly from adult populations of Eurasia.20 Among the 206 patients in the present study, four patients died of brain herniation, shock, multiple organ failure and pulmonary hemorrhage, respectively, with a mortality rate of 1.9%. In a Korean study of children HFRS, 4.8% of the patients (3/63) died of shock, respiratory failure and pulmonary hemorrhage.21 In a study from China, the case fatality rate of HFRS during the period 2004–2008 in patients primarily composed of adults is 1.17%.42 Consequently, the mortality rate in HFRS children appears not to be lower than in HFRS adults.
In view of the laboratory examinations, increased WBC and decreased PLT counts, elevated HB, hematuria and proteinuria were common in the children patients, especially in the severe and critical types of patients. Coagulation abnormalities were also common in the patients. Elevated Cr and UA and decreased eGFR were sequentially and significantly aggravated from mild, moderate, severe to critical type disease. Elevated ALT and AST levels and decreased ALB levels also exacerbated from mild, moderate, severe to critical types of disease. CK-MB was elevated in patients with severe and critical type disease. These findings are mostly similar with previous studies in HFRS children.21, 34 Importantly, this study showed that NEU%, PTA, PCT, BUN, and Ca2+ were independent risk factors for critical disease. NEU% are inflammatory indexes and their elevations are indicative of the severe systematic inflammation in the HFRS patients. PTA is an important coagulation index and its decrease is indicative of the abnormality of coagulation, a feature of HFRS.54 PCT is commonly used as a marker of bacterial infection but its elevation has been recorded in patients with HFRS, especially when the disease is caused by Dobrava virus or Hantaan virus infections.17, 55 Moreover, PCT levels at hospital admission have been demonstrated to be associated with the disease severity and patients' prognosis in adult HFRS patients caused by Hantaan virus.20 In the four died HFRS children of the present study, all of them had high levels of PCT at hospital admission. BUN is primarily a measure of serum urea, a by-product of the breakdown of blood, muscle and protein, that is eliminated by the kidney. High concentration of urea was demonstrated to increase the reactive oxygen species in mouse renal inner medullary (mIMCD3) cells in culture,56 indicating the potential effect of increased urea on kidney. Study in burn patients showed that BUN was independently associated with AKI developing.57 Therefore, the elevation of BUN in HFRS children may be resulted not only from the increased tissue breakdown in the disease but also from the kidney injury related to the disease per se and the effect of elevated urea on kidney. Interestingly, this study showed that, among the independent laboratory parameters associated with critical disease, BUN had the highest AUC for predicting the outcome and all the four children died of the disease had high levels of BUN at hospital admission, signifying the importance of this parameter in accurately evaluating the disease severity/prognosis. Hypocalcemia is common in critically ill patients.58 Consistent with our finding, a study in hospitalized patients showed that hypocalcemia on admission was associated with in-hospital mortality.59 Conceptually, eGFR is developed to describe kidney function in steady state and it is unsuitable to be used in AKI although we included eGFR in the analysis. In fact, eGFR was not identified to be independently associated with critical disease in this study.
Given the objectiveness of laboratory parameters and the easiness and convenience of nomogram in clinical practice,60 a nomogram based on BUN and PCT was constructed to visualize the probabilities of disease severity. The calibration curves for the outcome showed optimal agreements between the nomogram predictions and actual observation values, indicating that the goodness of fit was high. The Hosmer–Lemeshow tests showed that the degree of fit was not statistically significant, suggesting that the nomogram could accurately predict critical disease. Since there has been no simple and effective model to predict the severity/prognosis in pediatric patients with HFRS, the nomogram integrating routine laboratory parameters may be conveniently and objectively used for the disease severity prognostication and patient stratification in the management of HFRS children. This finding also provides information for the consultation with the parents/guardians in relation to the children's disease condition.
The present study has some limitations. First, all the pediatric patients with HFRS were enrolled from one hospital located in northwest of China and the study was a retrospective study. These natures of study may limit the generalization of the findings. Second, the nomogram may not be precisely used for mortality prediction, as this cohort only included four died patients. Therefore, further prospective studies are required to verify and extend our findings.
5 CONCLUSION
This study presented the clinical pictures of children HFRS in a large cohort of patients infected with Hantaan virus. Hydrothorax, cerebral edema/cerebral herniation and hypotension were independent clinical characteristics, and NEU%, PTA, PCT, BUN, and Ca2+ at hospital admission were independent laboratory factors associated with critical disease. A simple and easy-to-understand noninvasive nomogram integrating routine laboratory parameters (BUN and PCT) was constructed to visualize the probabilities of disease severity. These findings may serve as important references and method for disease severity evaluation and patient stratification in the management of children with HFRS related to Hantaan virus infection.
AUTHOR CONTRIBUTIONS
Ruina Li: Conceptualization, data curation, formal analysis, writing – original draft, writing – review and editing. Jingkang Sun: Conceptualization, data curation, formal analysis, writing – review and editing. Yuting Chen: Conceptualization, data curation, formal analysis, writing – review and editing. Qunying Han: Conceptualization, project administration and supervision, writing – review and editing. Zhengwen Liu: Conceptualization, project administration and supervision, writing – original draft, writing – review and editing. Xiude Fan: Data curation, formal analysis, writing – review and editing. Xiaoyun Wang: Data curation, formal analysis, writing – review and editing. Xiaoge Zhang: Data curation, formal analysis, writing – review and editing. Kun Zhang: Data curation, formal analysis, writing – review and editing.
ACKNOWLEDGMENT
We thank the medical record room staff of Xi'an Children's Hospital for their help during data collection of the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data are available upon request from the corresponding author upon reasonable request.




