Characterization and analysis of linear epitopes corresponding to SARS-CoV-2 outbreak in Jilin Province, China
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants have caused hundreds of thousands of deaths and shown serious social influence worldwide. Jilin Province, China, experienced the first wave of the outbreak from December 2020 to February 2021. Here, we analyzed the genomic characteristics of the SARS-CoV-2 outbreak in Jilin province using a phylogeographic tree and found that clinical isolates belonged to the B.1 lineage, which was considered to be the ancestral lineage. Several dominant SARS-CoV-2 specific linear B cell epitopes that reacted with the convalescent sera were also analysed and identified using a peptide microarray composed of S, M, and E proteins. Moreover, the serum of convalescent patients infected with SARS-CoV-2 showed neutralizing activity against four widely spreading SARS-CoV-2 variants; however, significant differences were observed in neutralizing activities against different SARS-CoV-2 variants. These data provide important information on genomic characteristics, linear epitopes, and neutralizing activity of SARS-CoV-2 outbreak in Jilin Province, China, which may aid in understanding disease patterns and regional aspects of the pandemic.
1 INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of the coronavirus disease 2019 (COVID-19) pandemic, has been rapidly spreading worldwide despite the progress in global vaccination. SARS-CoV-2 is a single-stranded positive-sense RNA virus belonging to the genus Betacoronavirus.1, 2 Its genome comprises 14 open reading frames (ORFs). Two-thirds of the genome encode 16 nonstructural proteins (NSP1–16) that compose the replicase/transcriptase complex (RTC). The remaining one-third of the genome encodes four structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N), and nine accessory proteins (ORF).3 The SARS-CoV-2 spike protein, located on the viral surface, mediates the binding of the virus to the human angiotensin-converting enzyme 2 (ACE2) receptor at the cell surface, thereby facilitating the invasion of the virus and infection of the host. In addition, the S protein is the main antigen component of all structural proteins of SARS-CoV-2 and hence, is the target of many vaccines and antibody drugs.4, 5
A large number of global SARS-CoV-2 infections have led to the emergence of various variants, notably Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2),6 and recently, Omicron (B.1.1.529)7 variants. These variants of concern (VOCs) are defined by mutations throughout the genome. Here, we only discuss mutations in the S protein. SARS-CoV-2 Alpha variant, which shows 13 mutations in its S gene, was initially reported in the United Kingdom (UK) in early December 2020.8, 9 The Alpha variant showed a 50% increase in infectivity as well as severity compared to the original strain based on hospitalizations and mortality rates.9 SARS-CoV-2 Beta (B.1.351) variant was first reported in South Africa in December 202010 with 10 mutation sites in its S gene. The Beta variant more frequently causes serious illness than other variants.11 The Gamma variant was first reported in December 2020 in Manaus, Brazil, with 11 mutation sites in the S gene. The Gamma variant shows approximately 2-fold more transmissible according to a local study in Manaus, Brazil, and is capable of evading approximately 32% of protective immunity compared to previous non-P.1 lineage diseases, leading to the possibility of reinfection.12 SARS-CoV-2 Delta (B.1.617.2) variant was first detected in India in October 2020 and became the most prevalent variant in European countries in mid-April 2021 according to European Centre for Disease Prevention and Control.13 It shows 10 mutation sites in its S gene. A new variant of SARS-CoV-2 B.1.1.529 (Omicron) was first reported to the World Health Organization (WHO) by South Africa on November 24, 2021.7 This variant carries an unusually high number of mutations, 32, in the S gene.14 SARS-CoV-2 variants have numerous mutations with the potential to increase transmissibility, confer resistance to therapeutics, or facilitate partial escape from infection- or vaccine-induced immunity.15, 16
Overall, multiple SARS-CoV-2 vaccines have shown protective efficacy against the original strains and VOCs.15, 17 Based on observational real-life data, vaccines seem to effectively prevent SARS-CoV-2 infection against the original strain and Alpha variant; however, Fiolet T et al. demonstrate reduced effectiveness against the Delta, Gamma, and Omicron strains.15 Despite significant progress in SARS-CoV-2 vaccine development, vaccine research needs to continue to improve, such as the types of antigens (epitopes) that elicit the production of robust neutralizing antibodies in most populations. B-cell epitopes are regions on the surface of an antigen that are recognized by specific antibodies. The antibodies bind to these regions and trigger an immune response.18 To identify these binding areas in the antigen sequence or structure is important for the development of synthetic vaccines,19, 20 diagnostic tests,21 and immunotherapeutics,22 particularly for protection against COVID-19 pandemic.
In this study, we collected sera from a convalescent SARS-CoV-2 cohort after the outbreak in Jilin Province, China. Using a peptide array comprising 19 Mer peptides of S, M, and E, we identified several dominant SARS-CoV-2 specific linear B cell epitopes that reacted with the convalescent sera. In addition, we tested the neutralizing antibody (NAb) activity of the serum of convalescent SARS-CoV-2-infected patients against four widely spreading SARS-CoV-2 variants (Alpha Variant (B.1.1.7), Beta (B.1.351), Gamma (p.1), and Delta (B.1.617.2)) and found significant differences in NAb against different SARS-CoV-2 variants. These data provide important information for the development of novel vaccines and antigens.
2 MATERIALS AND METHODS
2.1 Sample collection and ethics statement
A total of 62 patients who tested positive for SARS-CoV-2, as demonstrated by nasopharyngeal swab testing, and were admitted to the Infectious Diseases Hospital of Changchun City in Jilin Province, China were recruited for the study from January to February 2021. Demographic data, clinical and laboratory parameters, and clinical severity during the hospitalization period were retrieved from the patient records (Table 1). The patients were classified into three groups based on clinical severity: asymptomatic, mild, and severe. Patients were considered convalescent when negative results were obtained for three independent polymerase chain reaction (PCR)-based tests. In some cases, serum was collected twice or thrice during hospitalization. Blood samples from healthy individuals were collected before November 1 2018 as negative controls. Serum was collected from 70 healthcare workers 40 days after administration of the second dose of vaccine (Changchun Institute of Biological Products Co. Ltd.) and from 10 negative controls with a negative diagnosis of SARS-CoV-2 (who were not administered vaccination) to verify the significance and specificity of the epitope-antibody reactions. This study was approved by the Ethics Committee of the First Hospital of Jilin University (2020-691), and the procedures were performed in accordance with the approved guidelines.
| Patients (n = 62) | |
|---|---|
| Demographics | |
| Age median (range) | 59.5 (range 6–77) |
| Sex | |
| Male | 27 (43.5%) |
| Female | 35 (56.5%) |
| Comorbidities | |
| Diabetes | 12 (19.4%) |
| Heart disease | 12 (19.4%) |
| Hypertension | 11 (17.7%) |
| Renal disease | 3 (4.8%) |
| Gastrointestinal disease | 3 (4.8%) |
| Gout | 2 (3.2%) |
| History of pneumonia | 2 (3.2%) |
| HIV patients | 1 (1.6%) |
| Bladder cancer | 1 (1.6%) |
| Fever (yes/no) | 12 (19.4%) |
| Fatigue | 1 (1.6%) |
| Cough | 25 (40.3%) |
| Sputum production | 4 (6.5%) |
| Muscle ache | 1 (1.6%)1 |
| GI symptoms | 4 (6.5%) |
| Severity on admission | |
| Asymptomatic | 6 (9.7%) |
| Mild | 48 (77.4%) |
| Severity | 8 (12.9%) |
| Chest x-ray/CT findings (yes/no) | |
| Normal | 25 (40.3%) |
| Unilateral pneumonia | 4 (6.5%) |
| Bilateral pneumonia | 11 (17.7%) |
| Multiple mottling and ground-glass opacity | 22 (35.5%) |
2.2 Whole genome sequencing and genomics analysis
Total mRNA was extracted using a QIAamp Viral RNA Mini Kit (QIAGEN). Reverse transcription was performed using a Transcriptor First Strand cDNA Synthesis Kit (Roche) according to the manufacturer's instructions. The ULSEN® ultra-sensitive novel coronavirus whole-genome capture kit (V-090418-1, Mirco Future) was used for library construction and sequencing with Novogene Technology Co., Ltd. The low-quality reads and sequencing adapters were trimmed using Trimmomatic (v0.38) and then mapped to SARS-CoV-2 (NC_045512.2) genome using Burrows-Wheeler Aligner (BWA, v4.1.7). The GATK pipeline was then applied to call the variants using the following filter: QD < 2.0 || FS > 60.0 || SOR > 3.0 || MQRankSum < −12.5 || ReadPosRankSum < −8.0. The filtered variants were then applied to generate the consensus sequences with bcftools (v1.9), and the genome regions that were covered by less than 30 reads were masked by ‘N's. The phylogeny was constructed using the IQ-Tree (version 2.1.2)23 (with 10 000 bootstrap replicates) with the GTR model. iTOL (version 5)24 was used for phylogenetic tree visualization. Single nucleotide polymorphisms (SNPs) from each genome were called by Parsnp (version 1.2)25 from the harvest suite using NC_045512.2 as the reference genome.
2.3 Peptide array synthesis
Amino acids (length: 6–20 residues) covering the structural proteins S and N and the ectodomains M and E derived from the reference sequence (GenBank ID: NC_045512.2) were synthesized by GL Biochem and purified by high-performance liquid chromatography (HPLC) (Table 2). The peptide array was manufactured by Suzhou Epitope. The peptides were printed onto an activated integrated poly (dimethylsiloxane) membrane (iPDMS) (Epitope-Bio).
| Peptide | Sequence | Starting aa | Ending aa | |
|---|---|---|---|---|
| S1-1 | PINLVRDLPQGFSALEPL | 209 | 226 | [26] |
| S1-2 | YAWNRKRISNCVA | 351 | 363 | [27] |
| S1-3 | RQIAPGQTGKIADYNYKLPD | 408 | 427 | [27] |
| S1-4 | NCYFPLQSYGFQ | 487 | 498 | [27] |
| S1-5 | SYGFQPTNGVGYQ | 494 | 506 | [27] |
| S1-6 | ESNKKFLPFQ QFGRDIADTT | 554 | 573 | [28] |
| S1-7 | DAVRDPQTLE ILDITPCSFG | 574 | 593 | [28] |
| S1-8 | VSVITPGTNTSN | 595 | 606 | [26] |
| S1-9 | HADQLTPTWRVY | 625 | 636 | [27] |
| S1-10 | EHVNNSYECD IPIGAGICAS | 654 | 673 | [29] |
| S1-11 | DIADTT | 568 | 573 | [29] |
| S1-12 | GIAVEQDKNTQEVFAQVK | 769 | 786 | [26] |
| S2-1 | LPDPSKPSKR SFIEDLLFNK | 806 | 825 | [28] |
| S2-2 | VYDPLQPELDSF | 1137 | 1148 | [29] |
| S2-3 | DSFKEELDKY FKNHTSPDVD | 1146 | 1165 | [28] |
| E-1 | VFLLVTLAIL | 25 | 34 | [30] |
| M-1 | MADSNGTITV EELKKLLEQ | 1 | 19 | [28] |
| N-1 | KHIDAYKTFPPTEPKKDKKK | 355 | 374 | [31] |
| N-2 | NNAAIVLQLPQGTTLPKG | 153 | 170 | [26] |
- Note: aa: amino acid.
2.4 Initial serum screening
The sera samples were pretreated by heat inactivation at 56°C for 1 h and then diluted (1:200) and incubated with the peptide array at 37°C for 30 min. After washing thrice with wash buffer (Epitope-Bio), the peptide arrays were incubated with horseradish peroxidase (HRP)-conjugated goat anti-human IgG. The dots were visualized using SuperSignal™ Femto Maximum Sensitivity Substrate for Chemiluminescence (Thermo Fischer). The chemiluminescence intensity of each dot was converted to signal-to-noise ratio (SNR) using GenePix Pro 6.0 (Epitope-Bio) by subtracting the background intensity averaged from the intensity of the blank dots.
2.5 Cell culture and viruses
The Caco-2 cell line stably expressing N protein was maintained in Dulbecco's modified Eagle's medium (DMEM, HyClone) supplemented with 10% (v/v) fetal bovine serum (FBS, Biological Industries) and 50 IU/ml penicillin/streptomycin in a humidified 5% (v/v) CO2 incubator at 37°C. The Caco-2-N cell line tested negative for Mycoplasma. Recombinant SARS-CoV-2 GFP/ΔN virus and its mutants were constructed by Ding lab and have been described previously.32 Brief, cDNA (MN908947) of SARS-CoV-2 GFP/ΔN was synthesized from GenScript (the N gene was replaced with the green fluorescent protein [GFP] gene). The T7 promoter was introduced upstream of the 5′-untranslated region (UTR) of the SARS-CoV-2 genome. Other SARS-CoV-2 GFP/ΔN variants were constructed by mutating representative sites on the S protein of each mutant variant using SARS-CoV-2 GFP/ΔN as the template.
2.6 Virus neutralization assays
For neutralization assays, 3 × 105 Caco-2-N cells were seeded onto 24 well plates. SARS-CoV-2 GFP/ΔN virus or its mutants were included with the diluted sera (1:200 in DMEM supplemented with 2% FBS) at a multiplicity of infection (MOI) of 0.05 on Day 2 at 37°C for 30 min. The mixture was then added to an equal volume of cell medium (final dilution of serum was 1:400). Cells were collected for flow cytometry analysis to determine GFP expression on Day 4.
2.7 Statistical analysis
Data obtained from neutralization assays are presented as mean and standard deviation. Differences among groups were analysed by one-way analysis of variance (ANOVA) (Stata Corp.). NS, not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3 RESULTS
3.1 Whole genome analysis of SARS-CoV-2 during first wave of the outbreak in Jilin province
This non-interventional study enrolled 62 SARS-CoV-2 patients with a positive RT-PCR test performed with pharyngeal swabs. The median age of the patients was 45 years (range: 6–77 years). The majority of infections occurred in patients aged 20–65 years. The number of female patients was higher than that of male patients. The most common symptoms were fever (19.6%) and cough (40.3%). CT/X-ray evidence of the disease was remarkable with respect to bilateral pneumonia (37.56%). The rates of asymptomatic, mild, and severe SARS-CoV-2 were 9.7%, 77.4%, and 12.9%, respectively (Table 1).
In this study, we analyzed the whole genomes of SARS-CoV-2 in 62 clinical isolates collected from patients admitted to the Infectious Diseases Hospital of Changchun City during the first wave of the outbreak in Jilin Province (December 2020–February 2021), China. A total of 45 of 62 viral genomes were successfully assembled. Phylogenetic analysis was performed for 45 different SARS-CoV-2 sequences, and reference sequences of several variants were obtained from the GISAID database.33 All variants belonged to the B.1 lineage, which was considered the ancestral lineage compared to the VOCs and current prevalent lineages (such as Alpha, Beta, Gamma, Delta, and Omicron) (Figure 1A). The lineages likely originated during the Northern Italian outbreak in early 2020 according to CoV-Lineages.org Lineage Report.34
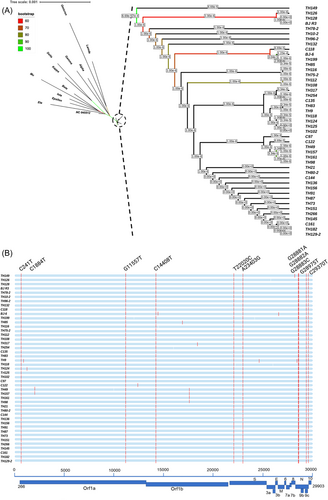
Ten nucleotide substitutions were observed in all 45 sequenced genomes: C241T (5′-UTR), G11557T (ORF1ab, E3764D), C14408 (NSP12, P4715L), T22020C (S, M153T), A23403G (S, D614G), G28881A, G28882A, and G28883C (S, R203K/G204R), G28975T (N, M234I), and C29370T at the 3′ end of the genome (Figure 1B). The high level of similarity at the SNP level indicates the closely related genetic nature of the strains prevalent during the outbreak. Most other point mutations were singletons, in which substitution was observed in only one or two strains and two site mutations, for instance, S T76I and S T1117I were found only in TH118 and TH9 strains, respectively.
3.2 Screening of dominant linear B-cell epitopes of SARS-CoV-2 by a peptide microarray
We designed 19 small peptides distributed in the entire S, N, the ectodomain of M, and E proteins of SARS-CoV-2 (Figure 2A), which were experimentally verified or predicted to have dominant linear B-cell epitopes.26, 28, 29 We used the peptide array to screen convalescent sera from 62 SARS-CoV-2 patients and 10 sera collected from healthy individuals with a negative diagnosis of SARS-CoV-2 and without vaccination. We unambiguously identified peptides that reacted with most of the sera based on the reaction heat map (Figure 2B). Peptides S1-2, S1-3, S1-4, S1-8, S1-9, and S2-2 showed little response to the serum of patients with SARS-CoV-2, while peptides S1-7, S2-1, S2-3, and N2 reacted with more than 35% SARS-CoV-2 sera (Figure 2B,C). Notably, S2-1 reacted with more than 15% of the SARS-CoV-2-negative control sera, indicating a nonspecific reaction.
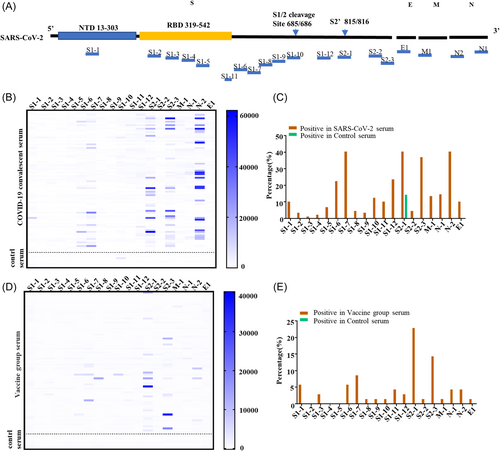
To verify the significance and specificity of the epitope-antibody reactions, we collected serum from 70 healthcare workers 40 days after receiving the second dose of vaccine, and 10 negative controls with a negative diagnosis of SARS-CoV-2, and without vaccination. The results showed that peptides S1-6, S1-7, S2-1, S2-3, and N2 had a significant reaction with the serum after vaccination (Figure 2D,E), which was consistent with the patient serum peptide results.
3.3 Antibody recognition activity of serum from various patients with dominant B cell linear epitopes
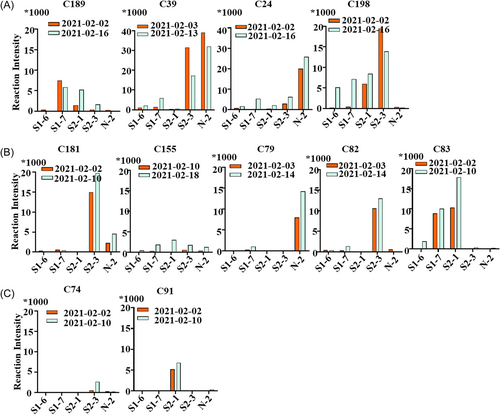
To analyse the antibody recognition activity in convalescent serum corresponding to different severities of COVID-19 patients, we selected serum samples from patients with severe (n = 4, C24, C39, C189, and C198), mild (n = 5, C79, C82, C83, C155, and C181), and asymptomatic infections (n = 2, C74, C91). The reaction intensity of the serum with screened B cell epitopes (S1-6, S1-7, S2-1, S2-3, N2) was determined. The sera from severe patients showed potent recognition intensity to B cell epitopes (Figure 3A), while the sera from mild symptoms or asymptomatic individuals showed less recognition ability (Figure 3B,C). In addition, no significant increase was observed in the recognition intensity in two or three blood samples collected approximately one week after the throat swabs tested negative (Figure 3A–C). Altogether, these results demonstrated that the severity of COVID-19 patients was positively correlated with the antibody recognition intensity and the response number of B cell epitopes. In addition, IgG antibody levels of COVID-19 individuals remained stable during the early stage of recovery without a rapid decline.
3.4 Neutralizing SARS-CoV-2 and variants infection by COVID-19 convalescent serum
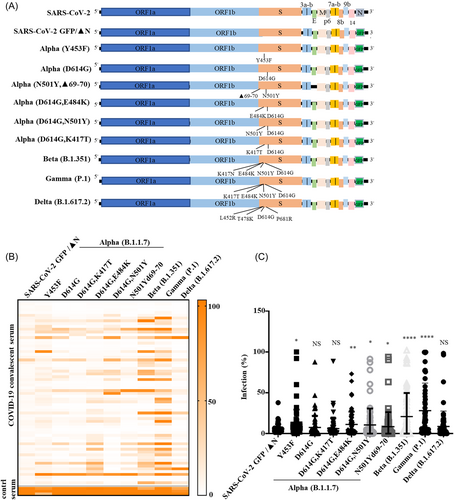
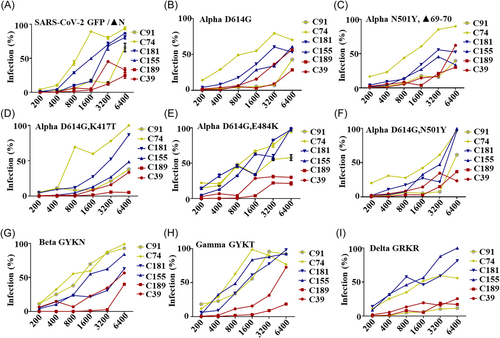
According to the World Health Organization classification, five main VOCs that have spread rapidly worldwide are Alpha (B.1.1.7), Beta (B.1.351), Gamma (p.1), Delta (B.1.617.2), and Omicron (B.1.1.529) variants.6 The latest VOC was first detected in Botswana in South Africa on 24 November 2021 when our study was already conducted; hence, it was not included in the current study. To verify the neutralization activity of the convalescent serum on these SARS-CoV-2 variants, the pseudo-type SARS-CoV-2 GFP/ΔN-trVLPs (the original strain (NC_045512.2) and its variants) were constructed and employed (Figure 4A). These trVLPs were only reproduced in cells expressing the N protein and can be used in a BSL-2 laboratory for high-throughput neutralization and antiviral screening.32 We detected whether antibodies in convalescent serum can neutralize various SARS-CoV-2 GFP/ΔN-trVLPs. The results showed that 62 COVID-19 convalescent sera had potent neutralization activity against the original strain SARS-CoV-2 GFP/ΔN-trVLP and several representative mutations (D614G; D614G, K417T) of the Alpha (B.1.1.7) variant. However, less neutralization activity against other mutations (Y453F; N501Y, Δ69-70; D614G, N501Y; and D614G, E484K) of the Alpha variant was observed (Figure 4B,C). Furthermore, these convalescent sera only showed weaker neutralizing activity against Beta (B.1.351) and Gamma (P.1) recombinant SARS-CoV-2 GFP/ΔN-trVLP. Interestingly, convalescent sera showed potent neutralizing activity against the Delta (B.1.617.2) variant which was endemic in the later stages (Figure 4B,C).
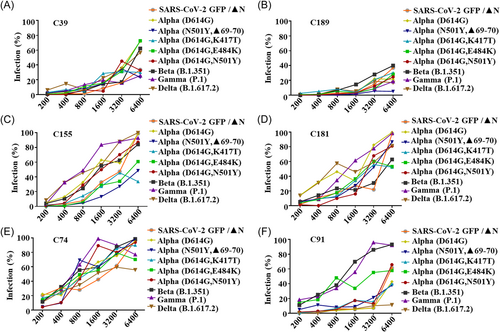
To study the neutralization response of serum from different disease progression for various SARS-CoV-2 variants, two serum samples were randomly selected from each of the following categories: asymptomatic (C74 and C91), mild (C155 and C181), and severe (C39 and C189). The serum of severe patients C39 and C189 exhibited strong neutralizing activity against all variants and a significant inhibition (>60%) even at a dilution of 1:3200 (Figures 5A–I and 6A,B). The serum of patients with mild symptoms, C155 and C181, exhibited a relatively weaker neutralizing activity compared with that with severe symptoms (Figures 5A–I and 6C,D). As expected, serum from the asymptomatic individual C74 had the weakest neutralizing activity against all variants (Figure 5A–I and Figure 6E). However, another serum sample from the asymptomatic individual C91 showed strong neutralizing activity against most of variants, except for Alpha (D614G, E484K), Beta (B.1.351), and Gamma (P.1), all of which comprised the E484K mutation in the spike protein (Figure 5A–I and Figure 6F). Taken together, the serum of severe patients showed potent neutralizing activity against different variants (Figure 6A,B), while the serum of mild patients showed relatively weak neutralizing activities (Figure 6C,D). Intestingly, one asymptomatic C91 serum neutralized recombinant viruses other than those harboring the E484k mutation (Figure 5F), indicating that the serum of C91 may harbor neutralization epitopes that were observed in this study.
4 DISCUSSION
The first wave of the SARS-CoV-2 outbreak was recorded in the Jilin Province from December 2020 to February 2021. At present, research on the SARS-CoV-2 strain that emerged in Jilin Province in December 2020 primarily focuses on clinical symptoms and epidemiological analysis.35-37 Our team reported the characteristics of whole genomic sequence of SARS-CoV-2 isolated in the Jilin Province for the first time. We analyzed 62 SARS-CoV-2 clinical isolates collected from patients during this outbreak, and 45 genomes were successfully assembled for whole-genome analysis. As expected, all strains showed a high level of genomic similarity, indicating that the outbreak likely originated from one source. We also used a peptide array to identify linear B cell epitopes that reacted to convalescent sera and assessed the neutralization activity of convalescent sera to several VOCs.
Linear B-cell epitope prediction research has steadily garnered significant interest ever since the first method was developed in 1981. B cell epitope identification with the help of an accurate prediction method can lead to an overall faster and cheaper vaccine design process, a crucial necessity in the COVID-19 era.38 Consequently, we selected 19 reported or predicted dominant linear B-cell epitopes and verified the neutralization ability of these epitopes in the serum of rehabilitated patients in the Jilin Province. Five dominant epitopes of B cells were screened, consistent with the experimental results reported by Yuan et al.28 Simultaneously, we examined the recognition activity of dominant B cell epitopes with the serum of 70 members of the medical staff after the second vaccination, and the results were consistent with those of the recovered serum. However, the reaction intensity of the vaccinated serum with B cell-dominant epitope was weaker than that of the recovered serum.
The spike (S) protein of SARS-CoV-2 decorates the viral surface and is an important target for the development of diagnostics and vaccine design.39 Mutations in the SARS-CoV-2 spike protein affect neutralization, thereby enhancing the escape of the virus from the immune reaction mediated by the vaccine.16 Therefore, we verified the neutralization of convalescent sera in Jilin province with different SARS-CoV-2 variant virus-like particles. The results showed that the neutralization effect on the original strain was significantly strong because the original strain had high homology with the SARS-CoV-2 sequence. However, the neutralizing activity of virus-like particles containing the Y453F, N501Y, and E484K mutations was relatively poor. Therefore, viruses with the Y453F, N501Y, and E484K mutations are more likely to demonstrate immune escape. The serum of patients with severe symptoms had the strongest neutralization ability against SARS-CoV-2 variants.
In summary, we identified dominant B-cell linear epitopes of SARS-CoV-2 in a COVID-19 cohort in Jilin Province. The inhibitory effect of antibodies in the rehabilitation serum on the infectivity of different SARS-CoV-2 variants was verified. These results provide a basis for the development of a new vaccine and the diagnosis and treatment of SARS-CoV-2.
AUTHOR CONTRIBUTIONS
Designed the study: W. Gao, W. Zhang. Conducted the study: W. Gao, Z. Li, Q. Guan, W. Cui, and B. Zheng. Clinical sample: G. Lv and J. Xu. Critical reagent: Q. Ding. Data analysis: W. Gao, Q. Guan, and W. Zhang. Manuscript draft: W. Gao, Q. Guan, and W. Zhang. Resources: W. Gao and W. Zhang.
ACKNOWLEDGMENTS
We thank the Infectious Diseases Hospital of Changchun City for the samples of COVID-19 patients as well as all medical staff who work in the frontline. We thank Professor Weifeng Shi (Medical School of Taishan, Shangdong) for useful discussions on the sequences of the 62 SARS-CoV-2 samples. This work was supported in part by funding from the National Key R&D Program of China (2021YFC2301900 and 2301904), National Natural Science Foundation of China (81930062 and 81672004 to ZWY, 31900457 and 82272304 to GWY), Science and Technology Department of Jilin Province (YDZJ202201ZYTS671, YDZJ202201ZYTS590), and Key Laboratory of Molecular Virology, Jilin Province (20102209).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




