Clinical features, therapeutic outcomes, and recovery period of long COVID
Abstract
To characterize the clinical features of long COVID, 286 patients who received care in our outpatient clinic for long COVID from May to December 2021 were surveyed. The recovery periods of each symptom and the key factors contributing to early recovery were statistically analysed. The median age of the patients was 35.8 years, with 137 men and 149 women. The median number of symptoms was 2.8. The most frequent symptoms were respiratory manifestations (52.1%), followed by fatigue (51.4%). Respiratory symptoms, fatigue, and headache/arthralgia were major complaints in the initial phase, whereas hair loss was a major complaint in the late phase, suggesting that the chief complaint of patients with long COVID may vary temporally. The best treatment outcome was observed for pulmonary symptoms, and hair loss had the worst outcome. COVID-19 severity, the number of manifestations, and the delay in starting treatment exerted a negative effect on the recovery period of long COVID. In addition, the smoking habit was an independent risk factor for slowing the recovery period from long COVID. This study provides insights into the clinical course of each manifestation and therapeutic options with a more certain future of long COVID to meet the unmet medical needs.
1 INTRODUCTION
Since the outbreak of COVID-19 in 2019, several epidemiological studies have found that COVID-19 subsequently causes sequelae, termed long COVID, in a substantial portion of patients.1-3 In a previous study from Italy, some symptoms persisted 60 days after COVID-19 onset in 87% of patients.1 Similarly, another large cohort study from China also showed that some complaints remain in 76% of patients with COVID-19 6 months after onset.4 Although a majority of patients with long COVID experience highly variable persistent signs and symptoms, such as chronic cough, breathlessness, fatigue, fever, and headache, with uncertain prospects for unknown periods, reliable evidence regarding the clinical features and valid management strategies for long COVID are still lacking.
First, the diagnostic criteria for long COVID differ, depending on the country. According to a summary of UK guidelines, long COVID is separately defined as acute COVID-19, ongoing symptomatic COVID-19, and post-COVID-19 syndrome based on the duration of symptoms for up to 4 weeks, from 4 weeks to up to 12 weeks and more than 12 weeks from the onset of COVID-19, respectively.5, 6 However, the post-COVID condition is considered in the US guidelines as persistent symptoms lasting over 4 weeks after the first infection.7, 8 Moreover, a certain standard for long COVID is currently unavailable in Japan.
Compared with the investigations of COVID-19 itself, the whole picture of long COVID, which is a multisystem disorder after an initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, is not less well understood. Therefore, the clinical course of long COVID has been unpredictable, and the ability to obtain relevant information regarding suitable approaches and a more certain future has not been feasible. In addition, the other problems of long COVID would be its low awareness and its difficulty in quantitative evaluation because the chief complaints of long COVID are mainly not objective but subjective symptoms. As a result, long COVID has already caused an untoward impact on communities, such as unfair discrimination from neighbors or retirement from a company, in addition to medical issues. In summary, an unmet medical need for a better understanding and characterization of long COVID outcomes and its multidisciplinary approach certainly exists.
To date, clinical evidence about the risk of prolongation, the pathological condition, and the effective treatment methods for long COVID has been particularly insufficient, even if some reports have documented the risks of long COVID appearance from COVID-19 infection.9-14 In these studies, age, body mass index (BMI), female sex, the severity of COVID-19, and multiple symptoms at COVID-19 onset were indicated as risk factors for long COVID development.
Taken together, we thus aimed to assess the profiles, including the clinical courses of each symptom, along with its future prediction, the possible risk factors for prolongation, and the specific treatment options in a cohort of patients with long COVID in a single Japanese medical center. These findings would help to summarize the standardized definition of long COVID and to achieve a global consensus.
2 MATERIALS AND METHODS
2.1 Study design and population
Two hundred eighty-six patients with previously confirmed SARS-CoV-2 infection by polymerase chain reaction(PCR) or antigen testing who were diagnosed with long COVID at our clinic between May 2021 and December 2021 were potentially eligible for inclusion in this study.
Clinical data, such as age, sex, BMI, and COVID-19 onset, PCR-positive date, consultation day, and laboratory data, including aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, of the selected patients, were collected and summarized in our database designed for this study. In addition, medical histories of diabetes mellitus, hypertension, hyperlipidemia, migraine, bronchial asthma, sleep apnea syndrome, and other conditions such as any cancer, Graves' disease, atrial fibrillation, cerebral infarction, depression, epilepsy, and so forth, as well as a history of smoking were similarly recorded based on self-reported information. For the evaluation of therapeutic efficacy, 29 censored patients were excluded from the analysis. A summary of the data collected from the study patients is presented in Table 1. The study protocol was approved by the ethics committee of UnMed Clinic Motomachi, Kanagawa, Japan (authorization number: UM22-01). The review board approved and waived the need for written informed consent from the participants due to the retrospective, noninterventional nature of this study. We have read the Declaration of Helsinki and have followed the recommended guidelines in this study.
| Parameters | Value |
|---|---|
| Age, mean (SD), years | 35.8 (11.8) |
| Sex, n (%) | |
| Men | 137 (47.9) |
| Women | 149 (52.1) |
| Body mass index, n (%) | |
| Underweight | 35 (12.2) |
| Normal weight | 190 (66.4) |
| Overweight | 45 (15.7) |
| Missing | 16 (5.6) |
| Smoking habits, n (%) | 64 (22.4) |
| Comorbidities, n (%) | |
| Any | 41 (14.3) |
| Respiratory | 16 (5.6) |
| Metabolic | 12 (4.2) |
| COVID-19 severity, n (%) | |
| Mild | 249 (87.1) |
| Moderate | 33 (11.5) |
| Severe | 4 (1.4) |
| Days from COVID-19 onset to first clinic visit, mean (SD) | 54.4 (41.1) |
| Symptoms, n (%) | |
| Fatigue | 147 (51.4) |
| Smell disturbance | 101 (35.3) |
| Fever | 61 (21.3) |
| Headache/joint pain | 98 (34.3) |
| Respiratory problems | 149 (52.1) |
| Digestive problems | 48 (16.8) |
| Palpitation | 31 (10.8) |
| Sleep disorder | 30 (10.5) |
| Hair loss | 76 (26.6) |
| Brain fog | 34 (11.9) |
| Dizziness | 26 (9.1) |
| Number of compliants, mean (SD) | 2.8 (1.6) |
| Blood examinations | |
| AST, mean (U/L) (SD) | 28.8 (26.0) |
| ALT, mean (U/L) (SD) | 30.8 (31.8) |
- Note: A summary of qualitative and quantitative variables in the study. Respiratory = bronchial asthma and sleep apnea syndrome. Metabolic = hypertension, hyperlipidemia, and diabetes.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
2.2 Therapeutic interventions for long COVID-related symptoms
Because herbaceous medications are generally agents that easily ameliorate a variety of systemic symptoms, including dysautonomia, they are actively administered to most patients, depending on the manifestations.
Therapeutic options for long COVID-related symptoms in our clinic are listed in Table 2. As previously reported, hochuekkito (TJ-41, 2.5 g three times daily), juzentaihoto (TJ-48, 2.5 g three times daily), and ninjinyoeito (TJ-108, 3.0 g three times daily) were administered for fatigue, and kakkonto (TJ-1, 2.5 g three times daily) and/or acetaminophen (500 mg orally as needed; not to exceed 3000 mg/day) were administered for fever, headache/joint pain, along with some other herbal drugs.15 For smell disorder, tokishakuyakusan (TJ-23, 2.5 g three times daily) was prescribed according to the clinical and basic evidence of its effectiveness for olfactory dysfunction.16, 17 In addition, rikkunshito (TJ-43, 2.5 g three times daily), bukuryoingohangekobokuto (TJ-116, 2.5 g three times daily), and hangeshashinto (TJ-14, 2.5 g three times daily) with a proton pump inhibitor (esomeprazole 10 mg or vonoprazan 10 mg), as an acid suppressant, and/or a dopamine-2 receptor antagonist (metoclopramide 100 mg orally as needed) were appropriately administered for digestive manifestations, such as nausea, stomachache, abdominal fullness, diarrhea, constipation.18, 19 On the basis of good results of a recent clinical trial in the UK, inhaled budesonide was actively used for pulmonary symptoms including cough, sore throat, sputum, dyspnea, chest pain with kikyoto (TJ-138, 2.5 g three times daily), carbocysteine (500 mg three times daily), and ambroxol hydrochloride (15 mg three times daily).20 For sleep disturbance, eszopiclone (1, 2, or 3 mg daily) and/or lemborexant (2.5, 5, or 10 mg daily), which are independent sleep aids, were palliatively introduced as needed. As a Japanese study previously successfully managed alopecia using saikokaryukotsuboreito (TJ-12, 2.5 g three times daily), this herbal drug was also selected to treat alopecia in the present study.21 Last, betahistine mesilate (12 mg orally as needed) and ryokeijyutsukanto (TJ-39, 2.5 g three times daily) were used to relieve dizziness symptoms. We were unable to provide appropriate care for brain fog or dysgeusia.
| Symptoms | Medications | |
|---|---|---|
| Herbal agents | The others | |
| Fatigue | Ninjinyoeito | |
| Juzendaihoto | ||
| Hochuekkito | ||
| Smell disorder | Tokishakuyakusan | |
| Fever | Kakkonto | Acetaminophen |
| Headache/Joint pain | Kakkonto | Acetaminophen |
| Goreisan | ||
| Chotosan | ||
| Respiratory problems | Kikyoto | Budesonide inhaler |
| Saibokuto | Carbocysteine | |
| shoseiryuto | Ambroxol hydrochloride | |
| Digestive manifestations | Rikkunshito | Mosapride citrate hydrate |
| Bukuryoingohangekobokuto | Proton pump inhibitor | |
| Hangeshashinto | scopolamine butylbromide | |
| Keishikashakuyakuto | Metoclopramide | |
| Daikencyuto | Probiotics | |
| Ramosetron hydrochloride | ||
| Palpitation | Saikokeishikankyoto | |
| Orengedokuto | ||
| Sleep disorder | Yokukansan | Eszopiclone |
| Lemborexant | ||
| Hair loss | Saikokaryukotsuboreito | |
| Dizziness | Ryokeijyutsukanto | Betahistine mesilate |
2.3 Outcomes
Primary outcomes were the clinical course with a futuristic overview, evaluation of the effectiveness of treatment for each manifestation, and risk factors affecting the recovery period of long COVID. Fatigue, smell disturbance, fever, headache/arthralgia, respiratory manifestations, digestive symptoms, circulatory disturbance, sleep disorder, hair loss, brain fog, and dizziness were separately assessed as distinct manifestations of long COVID in the analysis. For the validation of the recovery period, the clinical courses were divided into four groups: good (within a month or less), moderate (within 2 months), slow (within 3 months), and resistance (3 months or more). Clinical outcomes were measured at more than 3 months of follow-up, depending on the condition. The secondary outcome was the relationship between serum transaminase levels and fatigue determined by measuring blood samples from 164 selected patients.
2.4 Statistical analysis
Demographic and clinical data were extracted from electronic medical records. All statistical analyses were performed using SAS version 9.4 (SAS Institute). The χ2 test was used to compare distributions of categorical variables in Tables 3–6. Significant levels were set at p < 0.05. In the comparison of improvement of complaints after treatment, Bonferroni adjustment was conducted for each test separately to consider the number of significant tests undertaken.
| n, % | <1 month | 1 month | 2 months | 3+ months | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fatigue | 7 | 46.7% | 90 | 60.0% | 25 | 54.3% | 25 | 33.3% | 0.002 |
| Smell disturbance | 4 | 26.7% | 53 | 35.3% | 14 | 30.4% | 30 | 40.0% | 0.639 |
| Fever | 1 | 6.7% | 43 | 28.7% | 11 | 23.9% | 6 | 8.0% | 0.002 |
| Headache/joint pain | 5 | 33.3% | 63 | 42.0% | 17 | 37.0% | 13 | 17.3% | 0.003 |
| Respiratory problems | 11 | 73.3% | 102 | 68.0% | 20 | 43.5% | 16 | 21.3% | <0.001 |
| Digestive problems | 2 | 13.3% | 28 | 18.7% | 10 | 21.7% | 8 | 10.7% | 0.344 |
| Palpitation | 1 | 6.7% | 17 | 11.3% | 5 | 10.9% | 8 | 10.7% | 0.958 |
| Sleep disorder | 0 | 0.0% | 18 | 12.0% | 4 | 8.7% | 8 | 10.7% | 0.516 |
| Hair loss | 4 | 26.7% | 28 | 18.7% | 12 | 26.1% | 32 | 42.7% | 0.002 |
| Brain fog | 0 | 0.0% | 18 | 12.0% | 4 | 8.7% | 12 | 16.0% | 0.298 |
| Dizziness | 1 | 6.7% | 15 | 10.0% | 7 | 15.2% | 3 | 4.0% | 0.195 |
- Note: Analyzed by using the χ2 test.
| Overall (n = 257) | Severity | p | |||||
|---|---|---|---|---|---|---|---|
| All | Mild | Moderate/severe | |||||
| Good (2 weeks to 1 month) | 66 | 25.7% | 62 | 28.1% | 4 | 11.1% | 0.019 |
| Moderate (1–2 months) | 48 | 18.7% | 44 | 19.9% | 4 | 11.1% | |
| Slow (2–3 months) | 59 | 23.0% | 50 | 22.6% | 9 | 25.0% | |
| Resistance (3 months–) | 84 | 32.7% | 65 | 29.4% | 19 | 52.8% | |
| Overall (n = 257) | Number of compliant | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3+ | ||||||
| Good (2 weeks to 1 month) | 31 | 46.3% | 19 | 36.5% | 14 | 21.9% | 2 | 2.7% | <0.001 |
| Moderate (1–2 months) | 12 | 17.9% | 11 | 21.2% | 13 | 20.3% | 12 | 16.2% | |
| Slow (2–3 months) | 15 | 22.4% | 11 | 21.2% | 19 | 29.7% | 14 | 18.9% | |
| Resistance (3 months–) | 9 | 13.4% | 11 | 21.2% | 18 | 28.1% | 46 | 62.2% | |
| Overall (n = 257) | The duration between COVID-19 onset and start of treatment for long-COVID | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <1 month | 1 month | 2 months | 3+ months | ||||||
| Good (2 weeks to 1 month) | 4 | 30.8% | 38 | 28.1% | 10 | 23.8% | 14 | 20.9% | 0.014 |
| Moderate (1–2 months) | 4 | 30.8% | 30 | 22.2% | 1 | 2.4% | 13 | 19.4% | |
| Slow (2–3 months) | 3 | 23.1% | 22 | 16.3% | 11 | 26.2% | 23 | 34.3% | |
| Resistance (3 months–) | 2 | 15.4% | 45 | 33.3% | 20 | 47.6% | 17 | 25.4% | |
- Note: Analyzed by using the χ2 test.
| Overall (n = 257) | Smoking | Body mass index | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonsmoker | Smoker | p | Underweight | Normal weight | Overweight | |||||||
| Good (2 weeks to 1 month) | 56 | 28.3% | 10 | 16.9% | 0.021 | 10 | 31.3% | 49 | 26.8% | 7 | 16.7% | 0.151 |
| Moderate (1–2 months) | 39 | 19.7% | 9 | 15.3% | 5 | 15.6% | 34 | 18.6% | 9 | 21.4% | ||
| Slow (2–3 months) | 48 | 24.2% | 11 | 18.6% | 8 | 25.0% | 46 | 25.1% | 5 | 11.9% | ||
| Resistance (3 months–) | 55 | 27.8% | 29 | 49.2% | 9 | 28.1% | 54 | 29.5% | 21 | 50.0% | ||
| Overall (n = 257) | Sex | Age | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | p | <25 | 25–44 | 45+ | |||||||
| Good (2 weeks to 1 month) | 39 | 31.0% | 27 | 20.6% | 0.068 | 13 | 27.1% | 39 | 28.5% | 14 | 19.4% | 0.564 |
| Moderate (1–2 months) | 23 | 18.3% | 25 | 19.1% | 12 | 25.0% | 24 | 17.5% | 12 | 16.7% | ||
| Slow (2–3 months) | 21 | 16.7% | 38 | 29.0% | 10 | 20.8% | 32 | 23.4% | 17 | 23.6% | ||
| Resistance (3 months–) | 43 | 34.1% | 41 | 31.3% | 13 | 27.1% | 42 | 30.7% | 29 | 40.3% | ||
| Overall (n = 257) | Comorbidities | p | |||
|---|---|---|---|---|---|
| No | Any | ||||
| Good (2 weeks to 1 month) | 60 | 27.3% | 6 | 16.2% | 0.068 |
| Moderate (1–2 months) | 42 | 19.1% | 6 | 16.2% | |
| Slow (2–3 months) | 53 | 24.1% | 6 | 16.2% | |
| Resistance (3 months–) | 65 | 29.5% | 19 | 51.4% | |
- Note: Analyzed by using the χ2 test.
| Overall (n = 164) | AST (IU/L) | p | ALT (IU/L) | p | ||||
|---|---|---|---|---|---|---|---|---|
| Fatigue | –50 | 50–75 | 75– | –50 | 50-75 | 75– | ||
| Yes | 83 | 13 | 9 | 0.069 | 83 | 8 | 14 | 0.017 |
| No | 51 | 8 | 0 | 51 | 7 | 1 | ||
- Note: Analyzed by using the χ2 test.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
3 RESULTS
3.1 Patient characteristics
Two hundred eighty-six participants with long COVID completed the survey. Twenty-nine patients were excluded from the analysis of therapeutic effects because they were unable to fully follow their clinical courses. The demographic and clinical characteristics of the participants are summarized in Table 1. The median age of the enrolled patients was 35.8 (14–64) years, with 137 (47.9%) men and 149 (52.1%) women. The proportions of different BMI statuses among patients were 12.2% underweight, 66.4% normal weight, and 15.7% overweight. Sixty-four (22.4%) patients were current or former smokers, and 41 (14.3%) participants had any comorbidities, including 16 (5.6%) with respiratory diseases and 12 (4.2%) with metabolic diseases. Overall, 249 (87.1%) were outpatients with a mild illness, and 37 (12.9%) had a moderate (33 patients, 11.5%) or severe (4 patients, 1.4%) disease requiring hospitalization. The median duration from the onset of COVID-19 to initiation of treatment for long COVID was 54.4 (11–254) days. The median number of symptoms was 2.8 (1–9). The most frequent symptoms were respiratory manifestations (149/286, 52.1%), followed by fatigue (147/286, 51.4%), smell disorder (101/286, 35.3%), headache/joint pain (98/286, 34.3%), and hair loss (76/286, 26.6%). Sleep difficulties were ascertained in 30/286 (10.5%) individuals, whereas 34/286 (11.9%) patients presented cognitive disturbances, also called brain fog. Fortunately, no serious complications from our treatments were observed.
3.2 Chief complaints may change in the clinical course of long COVID
According to the χ2 test, respiratory symptoms tended to be a major complaint in the initial phase, followed by fatigue, fever, and headache/joint pain, whereas hair loss was the major complaint in the late phase (Table 3). Thus, our clinicians treating patients with long COVID should understand that the chief complaint of the patients may vary over time. Additionally, our data revealed no significant relationship between COVID-19 severity and the number of symptoms of long COVID (data not shown).
3.3 Variation in therapeutic efficacy among long COVID-related symptoms
Compared with previous studies, our data showed that the recovery period of pulmonary manifestations in patients with long COVID was undoubtedly shortened if they used inhaled budesonide.22-25 Using fatigue symptoms as the basis for comparison of the recovery period, pulmonary symptoms presented both a tendency towards early recovery and better therapeutic effect in most patients; subsequently, fever and headache/joint pain were comparatively well managed based on the rapid recovery in more patients (Figure 1). Conversely, hair loss was the worst symptom, with less quick recovery and more refractory cases, although hair loss tended to occur in the later phase. Brain fog, which has no effective therapeutic intervention, and smell disorder took second place in slow recovery (Figure 1).
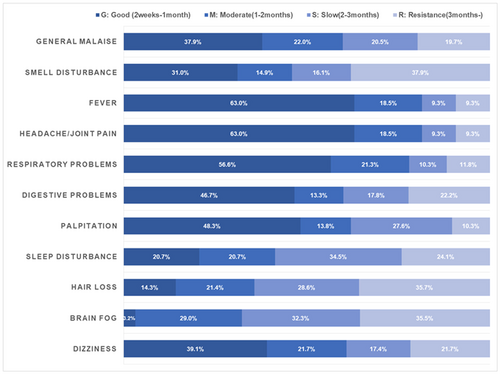
3.4 Independent risk factors influencing the recovery period of long COVID
As shown in Table 4, the duration of recovery from long COVID was significantly shorter in the mild COVID-19 group than in the moderate/severe COVID-19 group. Considering both our data and previous studies showing a significant difference in the association between COVID-19 severity and long COVID outcomes,12, 26-28 COVID-19 severity undoubtedly exerts a negative effect that cannot be ignored. Next, we focused on the effects of the number of complaints on the recovery period. Consistent with previous studies describing that multiple symptoms at the onset of COVID-19 were associated with long COVID occurrence,9, 14, 29 our results also revealed that the simple complaint group showed significantly faster improvement from long COVID compared with the multiple complaint group. An undeniable relationship between the number of manifestations and long COVID was observed. Moreover, earlier intervention for long COVID-related symptoms potentially led to more rapid recovery (Table 4). Thus, the sooner patients start treatment, the better the chances of recovery from long COVID.
3.5 Improvement of long COVID may be delayed by smoking
Strikingly, a smoking habit was an independent risk factor for slowing the recovery period from long COVID, whereas BMI, sex, age, and comorbidities were not (Table 5). Since smoking is a well-known major factor exacerbating COVID-19,30-32 our data support the medical rationale for promoting smoking cessation to prevent not only COVID-19 aggravation but also the delay in long COVID recovery.
3.6 Increased serum transaminase levels may be a surrogate marker of “fatigue”
Finally, we assessed the relationship between serum transaminase levels and fatigue symptoms in 164 patients from whom blood samples were selectively collected in case of blood test consent had been given. It is generally accepted that the liver is a major organ in the pathogenesis of fatigue because it regulates much of the storage, release, and production of substrate for energy generation,33 and it has been reported that ALT activity may be important not only as a marker of liver diseases but also as an indicator of general health.34 According to Specified Medical Checkups and Specified Health Guidance in Japan, ALT > 50 is a judgment value of recommendation for medical examination. Therefore, we selected the cutoff points of 50 and 75 IU/L from a clinical perspective. Interestingly, the correlation between fatigue symptoms and serum ALT levels was shown using a χ2 test, although AST levels showed no significant difference (Table 6). Because liver damage may occur during COVID-19 progression, regardless of pre-existing liver disease, our finding is consistent with that of a previous study.35 Namely, increased serum transaminase levels, especially ALT, may provide a convenient indication of fatigue.
4 DISCUSSION
To our knowledge, this study is the first to focus on the nature of long COVID-related symptoms and highlight the recovery period of systemic manifestations with prolonged risk factors. As shown in Table 1, the sampling of participants in this analysis ensures the validity of this work without any potential problems regarding selection bias. Consistent with previous studies, our data revealed that fatigue and respiratory symptoms are the two leading complaints at almost the same level.36-38
First, our data showed that long COVID has a dynamic nature of changeable manifestations throughout the clinical course in the majority of patients (Table 3), given the depressing impression that the clinical condition continuously improves and worsens over time, and the condition feels permanent.
Next, we assessed the therapeutic efficacy for each long COVID-related symptom. Strikingly, the effect of inhaled budesonide on pulmonary symptoms of long COVID contributes substantially to good recovery without any adverse events (Figure 1). Regardless of our data, the use of herbal drugs also depends on the management of long COVID-related manifestations, especially dysautonomia, among the limited therapeutic options (Table 2). Because the main application of herbaceous medications is basically alleviating symptoms, it can be reliable for unexplained diseases, similar to menopausal syndrome. Our physicians always have difficulty treating brain fog and dysgeusia due to the lack of definitive treatments. Because this existing condition would obviously affect the recovery period, the discovery of drugs for these symptoms is urgently needed. Intriguingly, a recent study from the UK showed brain structure changes with larger cognitive decline in 785 UK Biobank participants.39 However, challenges undoubtedly remain in this area. Similar to an existing study, hair loss occurred as a late-onset symptom, especially in females.3 This sex difference (data not shown) is probably because women usually have longer hair than men, easily attracting attention to their hair condition.
Next, independent risk factors affecting the recovery period from long COVID were evaluated by stratifying patients according to COVID-19 severity, the number of chief complaints, and the duration from the onset of COVID-19 to therapeutic intervention for long COVID. All of these factors exert a clinical effect on the recovery period of long COVID (Table 4), although the mechanism underlying their effects has not yet been elucidated. Our data suggested that patients with moderate or severe COVID-19 should receive more care for some persistent symptoms, and multiple complaints at long COVID onset are also an unwelcome sign, although the early onset of appropriate treatment might lead to better outcomes of COVID-19 and long COVID.
Then, a smoking habit was an independent risk factor for slowing the duration of recovery from long COVID (Table 5), suggesting a need for increased attention to smoking during the whole clinical course of COVID-19. On the other hand, sex (female), older age, and the presence of comorbidities were not independent risk factors for prolonged recovery in the analysis (Table 5), although several previous studies presented their negative effects on COVID-19.10, 12, 26, 36, 40-43 Of course, this finding must be confirmed in further studies; however, a prominent result was that smoking exerts a crucial effect on these infectious problems.
Finally, we found a significant relationship between increased serum transaminase, especially ALT, and fatigue symptoms in long COVID (Table 6). It was an unsurprising result based on a previous report,35 however ALT level was the only significant abnormal value in blood test of patients with long COVID in the analysis.
Taken together, we summarized the features of the changeable clinical course and proposed specific therapeutic options such as using inhaled budesonide and appropriate herbaceous medications with a clearer futuristic overview of long COVID in this study. However, this study has some possible limitations.
First, as the features of long COVID vary based on the variant, our data did not include the most recent COVID-19 variant, “Omicron.” We should always be conscious of the emergence of new variants and adjust to manage them accordingly. Second, the limited sample size and a single study site of the study participants would be a potential source of selection bias. A large cohort study in the UK demonstrated that the most common comorbidities were depression (22.1%), anxiety (20.3%), asthma (20.1%), eczema (19.5%), and hay fever (18.1%) in a total of 486 149 nonhospitalized long COVID patients including 12.2% of Asian participants.44 Finally, this study did not assess the effect of vaccination which is a potential confounding factor for long COVID, based on the lack of vaccination data gathering from all included patients. A recent study from Japan showed the effect of vaccination on changes in symptoms in patients with long COVID 45 and another one from the United States demonstrated that vaccination before COVID-19 infection confers only partial protection in the postacute phase of the disease.46 Further comprehensive investigations in larger populations will be required to fully understand the clinical profiles of long COVID.
5 CONCLUSION
A better understanding of the nature of long COVID can alleviate the physical and mental state of the patients. Our data underline the difference in recovery period among symptoms and the impact of a smoking habit on slowing the recovery period from long COVID. Regarding solutions for long COVID, wider recognition and continuous educational activities are equally essential as the elucidation of the pathological mechanism. We would be honored if our study provides some help to unravel the mechanism of this unexplained disease and meet the unmet medical needs.
AUTHOR CONTRIBUTIONS
Kazuki Takakura, Machi Suka, Mikio Kajihara, and Shigeo Koido conceived the study. Kazuki Takakura and Mikio Kajihara collected the qualitative data. Machi Suka analyzed the data. Kazuki Takakura wrote the first draft of the manuscript. Machi Suka and Mikio Kajihara reviewed and provided intellectual input on the draft and contributed to the writing of the manuscript. Shigeo Koido revised the manuscript based on input from Machi Suka and Mikio Kajihara. All authors reviewed and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




