A dual-targeting near-infrared biomimetic drug delivery system for HBV treatment
Liuxian Chen, Xinyun Jiang, Qiang Liu, and Zhenrong Tang have contributed equally to this work.
Abstract
Hepatitis B virus (HBV) infection is a serious global public health threat. It remains elusive to achieve a functional HBV cure with currently available antivirals. Herein, a photo-responsive delivery vehicle composed of Nd3+-sensitized core–shell upconversion nanoparticle (UCNP), mesoporous silica nanoparticle (MSN), antisense oligonucleotides (ASOs), and capsid-binding inhibitor C39 was established, which was named UMAC according to the initials of its components. Subsequently, the as-synthesized delivery vehicle was encapsulated by β- D-galactopyranoside (Gal) modified red blood cell (RBC) membrane vesicles, which enabled precise targeting of the liver cells (UMAC-M-Gal). Both in vitro and in vivo experiments demonstrated that this biomimetic system could successfully achieve controlled drug release under light conditions at 808 nm, leading to effective suppression of HBV replication in this dual-targeted therapeutic approach. Together, these results substantiate the system has huge prospects for application to achieve functional HBV cure, and provides a promising novel strategy for drug delivery.
1 INTRODUCTION
Hepatitis B virus infection caused by hepatitis B virus (HBV) leads to significant liver-related morbidity and mortality globally. According to the World Health Organization (WHO), nearly 300 million people worldwide suffer from long-term HBV infection, with more than 780 000 deaths associated with its complications (including liver cirrhosis and primary liver cancer) each year.1-3 The projected growth of the global market size for hepatitis B drugs will reach three billion dollars by 2024, placing an overwhelming burden on healthcare systems worldwide. Presently, due to the remaining elusive elimination of HBV covalently closed circular DNA (cccDNA), the therapy of chronic hepatitis B virus infection prefers a functional cure,4 which aims to sustainably reduce viral load and HBV surface protein antigen (HBsAg) from the circulation. In this respect, currently available drugs for clinical treatment of hepatitis B, such as interferons (IFNs) and nucleoside analogs (NUCs), can suppress the replication of hepatitis virus, slow down the progression of liver cirrhosis, and moderately reduce the morbidity of hepatocellular carcinoma (HCC). However, the lengthy treatment of these drugs is also accompanied by drug resistance and adverse effects such as bone marrow suppression.5 It is well-established that capsid inhibitors are crucial for the HBV life cycle and are a potential target for direct antiviral drugs for reducing HBV DNA.6 A recent report on a new type of capsid assembly regulator (CAM) revealed that a small-molecule C39 could prevent HBV DNA synthesis by affecting the normal assembly of HBV nucleocapsid.7 An increasing body of evidence from recently published studies revealed that antisense oligodeoxynucleotides (ASOs) can induce targeted cleavage of HBV mRNA with high specificity, resulting in decreased viral protein production.8
Significant inroads have been made to target the mechanisms underlying HBV persistence; the development of an anti-HBV therapy is no longer limited to acting directly on the virus itself but targets different stages in the virus life cycle by a multi-targeted therapeutic approach. Overwhelming evidence substantiates that this novel therapy concept seeking to treat complex diseases with a multidrug combination can effectively suppress HBV replication.9 However, neither highly negatively charged nucleic acids nor small-molecule drugs hardly enter mammalian cells without the aid of nanodelivery vehicles, imposing significant challenges for drug therapy. Moreover, injecting insoluble microparticles into the blood may trigger venous thromboembolism, coupled with the increase of potential systemic toxicity induced by the nonspecific distribution of the drug in the body, limiting their clinical application.
In recent years, drug delivery nanoparticles have been harnessed to enhance the delivery efficiency of drugs and decrease their systemic side effects.10, 11 Various nanoparticles have been used so far to target specific stages of the virus lifecycle, such as viral entry inhibitors,12, 13 viral DNA replication inhibitors,14 viral maturation inhibitors, and immunopotentiators15, 16 in antiviral therapy, whose antiviral activities have attracted much attention in recent years due to their efficiency and safety. Indeed, most of the nanoparticles cannot discriminate between abnormal cells and normal cells, usually leading to severe side effects, coupled with accidental release during delivery, so it remains a colossal challenge to rationally use drug delivery nanoparticles.17
Stimuli-responsive nanocarriers, a kind of smart nano vehicle, effectively reduce unexpected drug release and have sparked great attention in recent years.18-20 It has been established that photosensitive nanoparticles ensure controlled and efficient drug release at the diseased site temporally and spatially, standing out among stimuli-responsive polymeric materials due to their accessibility and noninvasive nature, which highlights their potential in the field of drug delivery systems and biomedical nanotechnology.21-24 The photo-responsive nanocarriers reported to date employ almost exclusively ultraviolet (UV) light-triggered transport mechanisms, whose application in vivo has been significantly limited due to phototoxicity and low penetrability of UV light.25 Since near-infrared (NIR) light can penetrate tissues with minimal damage to healthy tissues, it has attracted ongoing attention in noninvasive therapy and is widely used in biomedical research. There is ample evidence that upconversion nanoparticles, featuring advanced optical properties such as excellent dimensional stability and low toxicity, enable the conversion of multiphoton near-infrared light into single-photon localized ultraviolet/visible emission in vivo.26-30
In our previous study, upconversion nanoparticle (UCNP) was functionalized with polyacrylic acid (PAA) for surface modification; then, a photo-responsive CRISPR nano-vehicle was prepared through covalent binding to load Cas9/sgRNA, which indicated excellent controlled release properties induced by photo-irradiation.31 However, UCNP exhibited low drug-loading capacity, suggesting further optimization was required. The nanodelivery vehicles, which are on the basis of mesoporous silica nanoparticles (MSNs) and various small molecules comprising nucleic acid chains, have been demonstrated to possess excellent stimuli-responsive properties and controlled drug release.32-36 Although well-designed stimuli-responsive drug delivery systems displayed precise spatially controlled drug release, they still need improvements due to the lack of precise targeting capacity. Alternatively, nucleic acids on the surface of nanoparticles continuously exposed to complex physiological and heterogeneous environments are easily degraded by nucleases. Therefore, there is an urgent need to develop a novel delivery system with high targeting and high biocompatibility.
Due to the integration of the biomimetic features of cell membranes and the functional versatility of nanoparticles, cell membrane coating nanocarriers have emerged as a promising biomimetic approach to facilitate the delivery of various drugs, which have helped to achieve rapid developments in the field of drug delivery.37-41 Subsequently, for drugs to be delivered effectively into liver cells, β-D-Galactopyranoside (Gal) was used to modify cell membrane-derived vesicles, which targeted with high binding affinity the asialoglycoprotein receptor (ASGPR) specifically expressed on hepatocytes as a transmembrane protein.42-45 The as-obtained biomimetic cell-membrane-based nanocarriers possessed the ability to protect the nucleic acid from degradation, significantly improved the stability of nanoparticles and enabled accurate liver cell targeting.
As shown in Scheme 1, a novel UMAC photoresponsive nanoparticle, UCNP@MSN-ASO/C39, was successfully synthesized via mesoporous silica (MSN) surface modification to improve biocompatibility and load a large number of drugs. The as-designed DNA-containing PC group could be used to block the pores for the payload drug, which allowed the controlled release of the drug. ASO and viral capsid inhibitor C39 have been established to act on different targets in the lifecycle of HBV replication. This as-synthesized delivery system was finally encapsulated by Gal-modified RBC membrane vesicles, protecting the nucleic acid from degradation and promoting to precisely target the liver cells. After UMAC-M-Gal binding to ASGPR, a transmembrane protein specifically expressed on hepatocytes, receptor-mediated endocytosis occurred, resulting in accurate liver targeting. Under the 808 nm NIR radiation, the emission of UV light excited by UCNP enables the PC group to undergo photolysis, which triggers the release of ASO and C39, leading to a synergistic therapeutic effect to suppress HBV replication (Scheme 1).
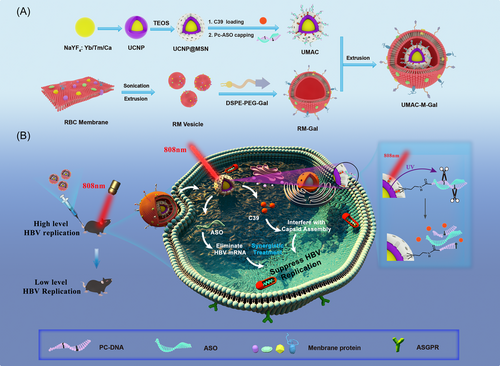
To spatially and temporally control drug release and precisely target the liver cells, we successfully constructed a biomimetic cell-membrane-based nano-drug delivery system, UMAC-M-Gal. Most critically, two types of drugs delivered by the nanocarrier yielded synergistic effects via targeting different stages in the virus life cycle and reducing the secretion of virus antigens to achieve a functional HBV cure.
2 RESULTS AND DISCUSSION
2.1 Synthesis and characterization of UMAC-M-Gal
The photoactivatable Pc-ASO was initially designed, as shown in Supporting Information: Figure S1. To examine the UV light-activatable property of Pc-ASO, we designed a Förster resonance energy transfer (FRET) pair as a fluorescent reporter by hybridizing a quencher (BHQ1)-bearing PcDNA strand and a FAM-labeled ASO strand (FAM-ASO). With the formation of a double-stranded structure, energy was transferred from FAM to the BHQ1, from which we could monitor the ultraviolet photoactivation process in real time (Supporting Information: Figure S1a). The fluorescence emission of FAM decreased significantly and almost disappeared, confirming Pc-ASO formation. With UV irradiation, the fluorescence emission of Pc-ASO gradually recovered, indicating the destruction of the FRET pair structure. Then FAM-ASO was released from the double-stranded structure due to the destruction of the PC group (Supporting Information: Figure S1b). As a negative control, we designed a single-strand DNA with the same sequence as PcDNA but without modifying the PC bonds. The fluorescence of the double-strand (nPc-ASO) was quenched by modified FRET, consistent with Pc-ASO results. However, the fluorescence emission of double-stranded structures did not increase with UV irradiation (Supporting Information: Figure S2b). Meanwhile, the specificity of PC bonds during agarose gel electrophoresis analysis (Supporting Information: Figure S2c,d), as in agreement with our previous FRET pair results, suggesting that the UV light-activatable property relies on the PC bonds.
We used an aliovalent Ca2+ ion doping strategy to enhance fluorescence intensity and improve the crystallization performance in the UV to visible light region, which could be used to respond to ultraviolet light. First, NaYF4:Yb/Tm/Ca UCNP with a diameter of ≈30 nm was prepared as described in our previous paper with slight modifications (Figure 1A and Supporting Information: Figure S3a).26 The hexagonal-phase core–shell NaYF4:Yb/Tm/Ca@NaYF4:Yb/Nd UCNPs were prepared with a mean diameter of 42 nm using the same method, in which the NaYF4:Yb/Tm/Ca core was encapsulated with an NaYF4:Yb/Nd shell through epitaxial growth (Figure 1B and Supporting Information: Figure S3b). The doping of Nd3+ as sensitizers reduced the strong water absorption of biological tissue at 980 nm and enabled the system to be excited at 808 nm to maximize the penetration depth in the tissue compared with the use of Yb3+ alone as the sensitizer (Supporting Information: Figure S4). Afterward, to prepare UCNP@MSN, oleic-acid-capped UCNP was modified and coated with mesoporous silica by random etching. A transmission electron microscope (TEM) (Figure 1C) showed that the particle size was approximately 57 nm. Meanwhile, we observed the mesoporous characteristics of UCNP@MSN (Figure 1C) and found that the mesoporous silicon layer was around 10 nm. Energy dispersive spectrome elemental analysis map and spectrum of UCNP@MSN (Figure 1D and Supporting Information: Figure S5) also confirmed successful preparation. High-resolution TEM (HRTEM) and X-ray diffraction revealed that the lattice spacing of UCNPs was 0.52 nm, and UCNPs had a hexagonal phase since their diffraction peaks corresponded to the hexagonal β-NaYF4 (JCPDS Card No. 28-1192) (Figure 1E and Supporting Information: Figure S6). Besides, an additional diffraction wide peak at 22° was observed in UCNP@MSN, indicating successful preparation of UCNP@MSN, attributed to the amorphous MSN of the sample. The pore characteristics of mesoporous-silica-coated UCNP were also measured by an automatic surface area and porosity analyzer, which showed that the specific surface area, pore size, and the total pore volume of UCNP@MSN were≈2.74 nm, 373.89 m2/g, and 0.352 cm3/g, respectively (Figure 1F and Supporting Information: Figure S7). The size of drug molecule C39 was estimated to be ≈1.49 nm (was analyzed by Chem3D software); therefore, the drug molecule C39 could be loaded into UCNP@MSN due to its appropriate pore size. Furthermore, the upconverting emission spectra of UCNP and UCNP@MSN (Figure 1H and Supporting Information: Figure S8) showed that UCNP@MSN could efficiently convert 808 nm NIR into light with emission peaks at 345 and 361 nm. Compared with UCNP, a moderate decrease in the luminescence emission was observed after being modified with the mesoporous silica layer, which could be due to the quenching effect of the surface of the silica shell.
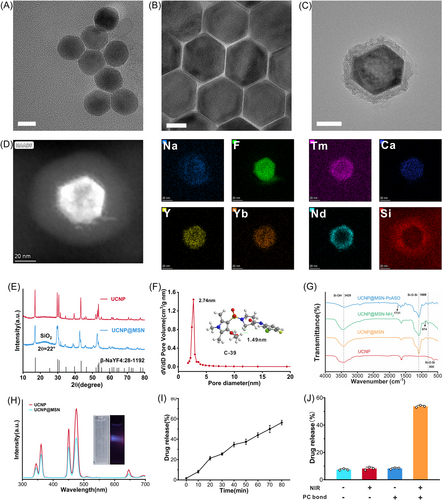
Next, FT-IR spectrometry was used to verify the functional modification of the nanoparticles. Fourier transform infrared spectra (FTIR) (Figure 1G) showed characteristic peaks at 3428, 1089, and 800 cm−1, which confirmed the successful surface modification of MSN. Compared with UCNP@MSN's FTIR results, an additional characteristic peak (NH2 ≈ 874 cm−1) was observed, indicating the successful graft of NH2. A COOH-modified single-stranded DNA was attached to UCNP@MSN via EDC/NHS condensation reaction between PcDNA and −NH2 in UCNP@MSN. After the ASO strand capping as a gatekeeper and C39 was loaded, the UV-Vis absorption spectrum suggested successful preparation (Supporting Information: Figure S9). Furthermore, C39 and ASO were successfully loaded on UCNP@MSN with an estimated loading content of 7.3 and 101.5 μmol/g UCNP@MSN, confirmed by the difference method (Supporting Information: Figure S10). The zeta potential of nanoparticles showed that the charge of UCNP@MSN-PcASO (UMA) decreased from −28 to −35 mV, which illustrated the successful modification of PcASO on the surface of UCNP@MSN (Figure 2E).
To probe the feasibility of controlled release of the drug delivery system UMAC, the C39 controlled releasing assay was performed in vitro with or without 808 nm NIR in phosphate-buffered saline (PBS), including detection of the time-dependent C39 drug-releasing curve (Figure 1I). In the UMAC without 808 nm NIR, only ≈8.1% of C39 was released at 48 h, indicating that the as-designed photoactivatable Pc-ASO structure blocked the mesoporous channels of MSN. Therefore, the drugs were contained in the nanoparticles. In sharp contrast, in the group treated with 808 nm NIR, the release of C39 significantly increased, with ≈55.7% of C39 released at 24 h (≈50.5% of C39 was released without DNA capping), showing that NIR significantly triggered the release of C39 (Figure 1J). The results showed that UMAC reached 56.8% drug release after continuous irradiation. Subsequently, the drug retention capacity of UMAC was evaluated by the drug leak rate in different solutions. After 24 h of incubation with no irradiation, minimal accidental drug release was observed, indicating that the as-prepared nanocomposite possessed good drug retention ability (Supporting Information: Figure S11).
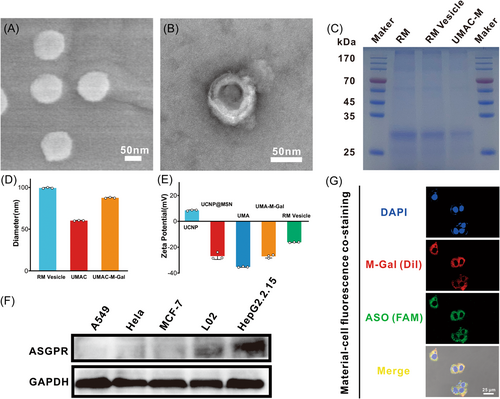
The membrane vesicles were obtained by sonication and then mechanical extrusion through a 200-nm membrane on an Avanti mini extruder, which was further used to encapsulate the USNP@MSN after post-modification with 4-aminophenyl β-d-galactopyranoside (Gal)-modified polyethylene glycol phospholipid (Gal-PEG-DSPE, Supporting Information: Figure S12) to form a Gal-PEG-RM wrapped UMAC (UMAC-M-Gal) (Figure 2A,B). SDS-PAGE protein analysis followed by coomassie brilliant blue staining demonstrated that RBC membrane proteins were widely retained by the nanocomposite (Figure 2C), showing a successful cell membrane coating on the UMAC. Dynamic light scattering (DLS) was further used to monitor nanocomposite size changes before and after the RBC membrane coating, which indicated that the size of UMAC-M-Gal was around 90 nm (Figure 2D) consistent with the results of the scanning electron microscope and transmission electron microscope. After the UMAC-vesicle fusion process, the hydrodynamic diameter of the resulting biomimetic nanoparticles increased by 30 nm (Figure 2D), and the ζ potential increased to the levels similar to RBC vesicles (Figure 2E). Moreover, we found that the obtained UMAC-M-Gal was stable in water, PBS, 100% fetal bovine serum (FBS), and 10% FBS-containing medium (Supporting Information: Figure S13a) and even possessed excellent stability for up to 14 days in FBS (Supporting Information: Figure S13b,c), assuring the feasibility of the biological experiments followed.
2.2 The evaluation of immune evasion in macrophages and pharmacokinetic study
After confirming the biomimetic membrane coating on the nanoparticles, we assessed the cellular uptake of the UMAC-M-Gal (ASO strand was labeled with FAM) in the liver-derived cells. Galactoside (Gal), modified on the biomimetic nanoparticles, could bind to the ASGPR protein (an endocytosis receptor) with high affinity and be specifically expressed in liver parenchyma cells. Before the experiments, the specific expression of ASGPR in liver cells was determined by western blot analysis, indicating that the receptor was almost exclusively expressed in hepatocytes compared with other tissue-derived cells (Figure 2F). Consequently, the follow-up experiments were performed using HepG2.2.15 cells or L02cells, which expressed more ASGPR protein on the cell membranes. Next, we tested HepG2.2.15 cells by incubating them with UMAC-M-Gal (RM-Gal labeled with Dil and ASO labeled with FAM). we observed the majority of signal for Dil (red) overlapped with FAM signal (green) surrounding the nuclei, which confirmed the successful coating of RBC membrane on the UMAC surface (Figure 2G).
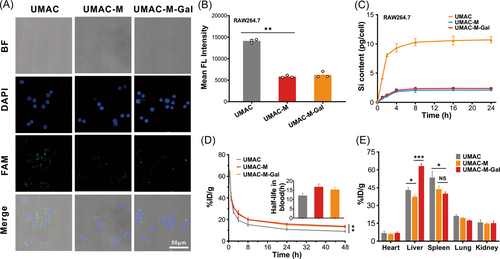
To validate the ability of immune escape, RAW 264.7 murine macrophage-like cells were used to examine the antiphagocytosis capability of UMAC-M-Gal. The cells were incubated with various nanoparticles and then imaged using a confocal laser scanning microscope. We found that the group incubated with UMAC showed brighter green fluorescence than the group incubated with UMAC-M and UMAC-M-Gal (Figure 3A), reflecting the idea that the encapsulation of erythrocyte membrane could effectively reduce the immune clearance. Further, a macrophage uptake test was performed using inductively coupled plasma mass spectrometry (ICP-MS) to detect the content of Si within the cytoplasm, showing that RBC membrane-coated biomimetic nanoparticles (UMAC-M and UMAC-M-Gal) exhibited much lower uptake compared with the uncoated nanoparticles (Figure 3C). As mentioned above, the protection of cell membrane could endow the nanoparticles with the ability to resist immune clearance. During in vivo studies on blood samples at different time points after intravenous injection, we detected the silicon content by ICP-MS, which indicated that the blood retention of the particles coated in the cell membrane was significantly enhanced (Figure 3D). It has been established that the reticuloendothelial system (RES) contains phagocytes mainly distributed in the liver and spleen, which resulted in the accumulation of substantial nanoparticles during treatment. We found that significant Si accumulation of UMAC-M in both organs decreased significantly, which indicated that the cell membrane wrapping could reduce the uptake of RES to some extent (Figure 3E).
2.3 NIR light triggered release of ASO in vitro
Next, to verify that the drug delivery system possessed the potential for temporal and spatial controlled-release capacity, HepG2.2.15 cells were incubated with a medium containing FRET pair-labeled UMAC-M-Gal (PcASO) or UMAC-M-Gal (nPcASO). After coculturing for 3 h, an 808 nm CW (1.5 W/cm2) laser was used to treat the cells. We found almost no fluorescent signal in cells without irradiation, whereas significantly stronger green fluorescence was detected with NIR irradiation, which indicated that NIR triggered liberation of the ASO-FAM strand (Supporting Information: Figure S14a). Flow cytometry quantitation also showed consistent results (Supporting Information: Figure S14b). In contrast, the green fluorescence intensity in HepG2.2.15 cells incubated with FRET pair-labeled UMAC-M-Gal (nPcASO) was barely changed under irradiation (Supporting Information: Figure S15a,b), suggesting that the PC bond was crucial for the biomimetic drug delivery system.
2.4 The evaluation of cellular uptake and liver targeting
A weak green fluorescent signal was observed upon incubation with a free ASO strand as naked DNA could not penetrate the cell membrane. In stark contrast, the cells incubated with UMAC displayed a stronger fluorescence signal, indicating that the presence of nanoparticles could promote the entry of ASO strands into cells. Concurrently, both confocal laser scanning microscopy (CLSM) analysis and flow cytometry demonstrated that UMAC-M-Gal possessed the highest internalization efficiency in the whole group, which indicated that the biomimetic membrane vesicles modified with Gal molecules were crucial for improving the internalization of the UMAC drug delivery system (Figure 4A and Supporting Information: Figure S16). Almost all fluorescence signals were located within the intracellular region, meaning that the UMAC-M-Gal was taken up via the endocytic pathway rather than physical adsorption. Subsequently, we evaluated the internalization of UMAC-M-Gal in L02 cells by co-localization study and found that after 3 h of incubation, UMAC-M-Gal showed good co-localization with lysosomes and endosomes stained by Lysotracker Red, indicating that UMAC-M-Gal was more likely endocytosed into the cells (Supporting Information: Figure S17). Additionally, to further study the key role of Gal in hepatocyte targeting, an antibody blocking test was performed on L02 cells by using an anti-ASGPR receptor antibody. We found that UMAC-M-Gal possessed a weaker fluorescence signal in the cytoplasm after blocking (Supporting Information: Figure S18).
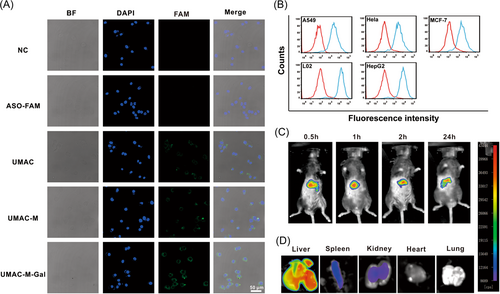
Ultimately, to evaluate the targeting capability of biomimetic nanoparticles, a follow-up study was performed using CLSM, flow cytometry, and ICP-MS. A confocal co-localization assay was performed in various non-liver cell lines (A549, HeLa, and MCF7 cells), indicating significantly weaker fluorescent accumulation of UMAC-M-Gal, compared with the intensive fluorescent signal of the L02 and HepG2.2.15 cells (Supporting Information: Figure S19a). Flow cytometry analysis showed that the amount of UMAC-M-Gal bound to the liver cells was significantly higher than non-liver cell lines after incubation (Figure 4B and Supporting Information: Figure S19b). Moreover, the ICP-MS results confirmed that the biomimetic nanoparticles modified with Gal demonstrated significantly more silicon accumulation (Supporting Information: Figure S19c). These results indicate that UMAC-M-Gal possessed a high hepatocyte targeting specificity. Afterward, the real-time biodistribution of UMAC-M-Gal in vivo was tracked using a modified in vivo fluorescence imaging system. At 24 h after the injection, the biomimetic drug delivery system UMAC-M-Gal, labeled with the lipophilic fluorescent dye DiR, detected massive accumulation in the liver (Figure 4C,D). To study the biodistribution of different nanoparticles in vivo, the number of nanoparticles in the major organs of the killed mice and blood samples were accurately analyzed by detecting the Si content. Consistent with the fluorescence imaging in vivo, nanoparticles modified with Gal possessed a good targeting ability to liver. Concurrently, the low accumulation of UMAC-M and UMAC-M-Gal in the spleen corroborated the encapsulation of erythrocyte membrane could endow nanoparticles with immune escape ability. Moreover, the high accumulation of UMAC-M-Gal in the liver was attributed to the targeting ability of Gal.
2.5 NIR light-triggered anti-HBV effects in vitro
To investigate the molecular biological effects of UMAC-M-Gal nanoparticles in a complex physiological environment, we investigated the capacity of the biomimetic system to suppress intracellular hepatitis B virus replication. Before that, considering the thermal efficiency of the 808 nm NIR and the NIR-responsive photothermal property of nanoparticles, we assessed temperature changes by irradiating the DMEM containing 10% FBS and the UMAC-M-Gal solution, which evaluated the risk of NIR laser-induced thermal effect. A high thermal effect was barely detected even at a concentration of 800 μg/ml and 5 W/cm2 NIR irradiation up to 10 min (Supporting Information: Figure S20). L02 and HepG2.2.15 cells, a normal liver cell model and a cell model undergoing the integration of the HBV genome into the chromosomal DNA, were used to assess the in vitro cell viability of UMAC-M-Gal, suggesting negligible potential cytotoxic effects of nanoparticles. First, the cells were divided into four groups: the first group determined the effects of different concentrations of nanoparticles on cell viability, the second group was treated with 808 nm irradiation for 30 min, the third group was treated with 808 nm irradiation with different power density, the fourth group assessed the toxic effect of various concentrations of UMAC-M-Gal on cells under a fixed laser power and irradiation time. Interestingly, even when the laser power reached 5 W/cm2 for 5 min or 1 W/cm2 for 30 min, the cells in the irradiation group did not exhibit a significant cytotoxic effect, suggesting that near-infrared laser did not affect the survival rate of liver cells (Supporting Information: Figure S21b,c). Furthermore, the results suggested that over 90% of HepG2.2.15 and L02 cells survived after incubating with 800 μg/ml UMAC-M-Gal and 1.0 W/cm2 irradiation for 10 min, showing the high biocompatibility and their potential value in further biomedical applications (Supporting Information: Figure S21d).
The following experiments were performed to evaluate the inhibitory effect of UMAC-M-Gal on HBV replication in vitro. First, we determined the difference in therapeutic efficacy between drug treatment and biomimetic drug delivery systems. We found that the combination of ASO and C39 synergistically potentiated the inhibitory effect against HBV (Figure 5D–G). Meanwhile, we observed that the bionic drug delivery system significantly enhanced the antiviral ability. Subsequently, we verified the manipulative role of near-infrared light and PC bonds during the inhibition of HBV replication. We measured HBV 3.5 kb RNA and intracellular HBV DNA by real-time quantitative reverse transcription polymerase chain reaction and real-time quantitative polymerase chain reaction. At the same time, the level of HBV virus antigens (such as HBsAg and HBeAg) in supernatants was detected with an enzyme-linked immunosorbent assay. Due to the absence of PC bonds, UMAC-M-Gal had no obvious effect on HBV replication with or without NIR irradiation, which could be induced by the inhibition of drug release by hybridization with nPcDNA (Figure 5H,I). In contrast, the HBV 3.5 kb RNA, intracellular HBV DNA, and HBV viral antigens of the group incubated with nanocomposites modified with PC bonds decreased significantly under NIR irradiation, confirming that the observed HBV inhibitory activity of UMAC-M-Gal was indeed induced by NIR-mediated activation (Figure 5J,K).
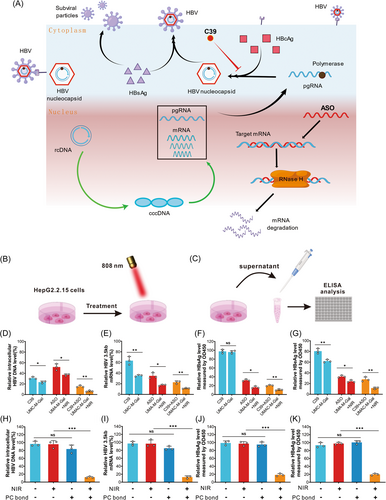
Finally, we determined the effect of enhancement of the biofilm and Gal on the ability of the biomimetic nano-delivery system to inhibit HBV replication. We found that the encapsulation of RBC membrane vesicles and the modification of Gal could promote the delivery of the biomimetic nanoparticles. Nevertheless, the effect was not significant (Supporting Information: Figure S22) and should be further verified in vivo experiments.
2.6 NIR light-triggered Anti-HBV Effects in vivo
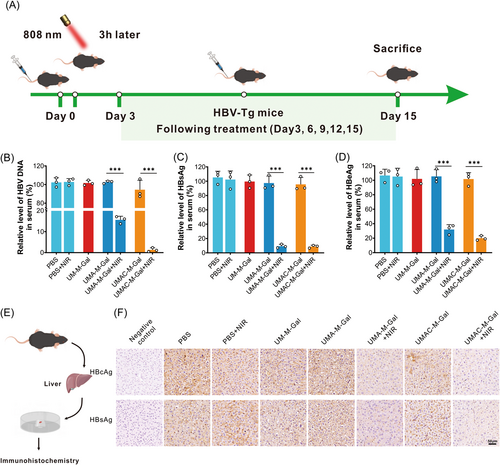
HBV-transgenic mice (HBV-Tg C57 BL/6), encoding a 1.2-overlength copy of the HBV genome (serotype awy), were used as the animal model to evaluate the ability of the UMAC-M-Gal drug delivery system to suppress HBV replication in vivo. These transgenic mice synthesize high concentrations of viral particles and HBsAg, pre-S1, and HBeAg, which should be used for detailed studies of the replication and expression of HBV and for pathological studies of hepatitis. Before this, to assess the further biomedical application of the as-obtained nano-system, a hemolysis assay was performed, suggesting negligible hemolysis capacity of the nanoparticles (Supporting Information: Figure S23).
As shown in Figure 6A, the parameters related to HBV replication in the HBV-Tg mice serum declined strikingly on Day 15 after treatment with biomimetic nanoparticles and illumination. Among them, the serum HBV DNA in the mice treated with NPs loaded with ASO only decreased to 15% under near-infrared irradiation, and the mice treated with NPs loaded with ASO and C39 even decreased to 1.3% (Figure 6B), indicating the potential for a functional cure. Analogously, the detected concentrations of HBsAg and HBeAg were predominantly consistent with the above after treatment with the biomimetic nanoparticles and NIR but was not observed decline in the transgenic mice exposed to NIR irradiation only (Figure 6C,D). Furthermore, immunohistochemical (IHC) staining was used to investigate the expression of viral antigen (HBcAg and HBsAg) in the HBV transgenic mice liver (Figure 6F), showing a barely detectable signal on Day 15 after treatment with both UMAC-M-Gal nanoparticles and NIR irradiation. Moreover, histopathological analysis (H&E staining) of the organs suggested no organ injury, while a definite therapeutic effect in IHC staining was detected in the liver after treatment (Supporting Information: Figure S24). Meanwhile, there were no significant changes in body weight during treatment (Supporting Information: Figure S25a) and blood biochemical parameters after treatment (Supporting Information: Figure S25b–d). These experimental data confirmed that treatment with UMAC-M-Gal plus NIR irradiation possessed good biocompatibility and properties without systemic toxicity in vivo, exhibiting tremendous potential utility to achieve a functional cure for chronic HBV infection.
3 CONCLUSIONS
In this experiment, considering the various effects on experimental mice, we did not use autologous RBC membranes. Cell membrane bionanotechnology is an emerging and promising technique in disease treatment,46 if there was an opportunity to be exploited for the treatment of human diseases in the future, autologous RBC membranes would represent a better alternative in theory. 808 nm NIR light, with its low energy, has a low thermal effect, and is relatively safe for human use within the safe power.47, 48 In general, the penetration depth of NIR light in tissues could achieve millimeter-order (even up to 3.2 cm).49, 50 For several organs, however, the tissue penetration depth and applicable NIR power are slightly insufficient for the human body. Therefore, there is still a long way to go before the application of NIR light in clinical practice.
In conclusion, we proposed a novel photo-induced co-delivery strategy to achieve integrated treatment of HBV. First, by exploiting the immune escape of cell membrane and the targeting capability of Gal, we demonstrated the successful delivery of the nano-system to hepatocyte. Under near-infrared irradiation, the ultraviolet light emitted by UCNP successfully triggered the release of ASO and C39, which was attributed to the special light-induced properties of the PC bond. Notably, our drug delivery system achieved a functional cure of HBV in vitro and in vivo. Indeed, these findings provide a delivery strategy for treating other diseases (especially tumors), and this novel means of controlled release offers an advanced strategy and a neoteric insight for drug delivery.
AUTHOR CONTRIBUTIONS
Liuxian Chen and Xinyun Jiang performed the in vitro assays. Qiang Liu and Zhenrong Tang did the in vivo studies. Dan Wang, Zheng Xiang, and Shengchun Liu analyzed the data, Liuxian Chen wrote the manuscript. Tang Hua designed this study and reviewed the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to express our gratitude to all those who supported the study. We thank Figdraw for drawing Figure 5A. This study was supported by the Key Laboratory of Infectious Diseases, CQMU (202004) and Postgraduate scientific research innovation project of Chongqing, China (CYS21245).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




