Transplacental transmission of SARS-CoV-2 immunoglobulin G antibody to infants from maternal COVID-19 vaccine immunization before pregnancy
Abstract
The coronavirus disease 2019 (COVID-19) vaccine generates functional antibodies in maternal circulation that are detectable in infants, while the information is restricted to the usage of COVID-19 vaccine during pregnancy. In this study, we aimed to evaluate the effect of maternal COVID-19 vaccines before pregnancy. Infants were included from mothers with no inactivated COVID-19 vaccine, 1-, 2-, and 3-dose before pregnancy, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) antibodies were tested. Comparative analysis was done between the groups. A total of 130 infants were enrolled in the study. Significantly higher levels of SARS-CoV-2 IgG antibodies in infants born to mothers with 3-dose COVID-19 vaccine before pregnancy compared with 1- and 2-dose groups (p < 0.0001). The levels of antibodies decreased significantly with age in infants born to mothers with the 3-dose COVID-19 vaccine before pregnancy (r = −0.338, p = 0.035), and it was still higher than that 2-dose COVID-19 vaccine group. The maternal SARS-CoV-2 antibodies produced from the inactivated COVID-19 vaccine before pregnancy can be transferred to newborns via the placenta. Maternal immunization with 3-dose of the COVID-19 vaccine before pregnancy could be more beneficial for both mothers and infants.
1 INTRODUCTION
Maternal immunization is thought to be an important way for mothers to protect themselves and their babies. The administration of the coronavirus disease 2019 (COVID-19) vaccine during pregnancy generates functional severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific antibodies in maternal circulation, and can transfer across the placenta barrier.1-3 The passive transfer antibodies provided by maternal immunization during pregnancy could be effective in reducing the risk of COVID-19-related hospitalization in infants younger than 6 months of age,4, 5 but the effect of maternal COVID-19 vaccines before pregnancy on infants is unknown. Some kinds of COVID-19 vaccines are strongly recommended to women who are trying to conceive.6 Thus, additional information of the effect of inactivated COVID-19 vaccine before pregnancy on infants is needed.
COVID-19 vaccines are the safest and most effective way to offer strong protection against severe illness from SARS-CoV-2 infection.7-11 Although the possibility of intrauterine vertical transmission of SARS-CoV-2 is still controversial in the literature,12-14 previous studies have demonstrated that pregnancy and infants with SARS-CoV-2 infection are at increased risk for severe disease.7, 10 Recently, Rasmussen and Jamieson15 infer that COVID-19 vaccination during pregnancy is a “two for the price of one deal.” Inactivated COVID-19 vaccine has been administered in more than 40 countries,16 and is one of the most widely administered vaccine types in populations other than pregnant women. Understanding whether maternal immunization before pregnancy affects subsequence placental antibody is critical to guiding vaccine recommendations for the special population. In this study, we aimed to evaluate SARS-CoV-2 immunoglobulin G (IgG) antibody levels in infants from mothers with inactivated COVID-19 vaccine before pregnancy.
2 METHODS
2.1 Study population
The study was conducted in the National Clinical Research Center for Child Health and Disorders in China (Children's Hospital of Chongqing Medical University) between July 2, 2022, and September 28, 2022. At that moment in time and a long time before, the Chinese mainland had the lowest rate of COVID-19 infections and COVID-19-related mortality in the world. Thus, women would be an excellent population to study the effect of maternal COVID-19 vaccination before pregnancy on infants, minimizing the influence caused by SARS-CoV-2 infection. Additionally, all inactivated COVID-19 vaccine is stored, managed, and used in accordance with national laws and regulations.17, 18 This study was approved by the Ethics Review Committee of Children's Hospital of Chongqing Medical University.
2.2 Sample collection
To test the transmission of SARS-CoV-2 IgG antibodies from mother to infant during pregnancy, we included infants born to mothers that did not receive any inactivated, 1-, 2-, or 3-dose COVID-19 vaccine before pregnancy. The main exclusion criteria included a history of SARS-CoV-2 infection, and a recent history (within 14 days before enrollment) of travel with or exposure to infected individuals, born to mothers with COVID-19 vaccination during pregnancy. The diagnosis of SARS-CoV-2 infection is based on the quantitative nucleic acid testing of nasopharyngeal swab samples (Daan Gene) in the laboratory. The total serum IgG value in infants was detected with an enzyme-linked immunosorbent assay (ELISA) kit (Jonln) according to the instructions.
The demographic information of mothers and infants were recorded after enrollment. A portion of the residual serum is collected within 2 h of completion of the clinical program testing of the infant blood sample and stored at −80°C until use.
2.3 Quantification of SARS-CoV-2 IgG antibodies
Antibody levels against the SARS-CoV-2 spike protein in infants were quantified using an ELISA. Assays were performed in 96-well plates coated with 100 µl of recombinant S protein (residues 16-685, Cat. #40591-V08H; Sino Biological) diluted to 1 µg/ml in phosphate-buffered saline (PBS), and plates were incubated at 4°C overnight. The plates were washed with PBS-T (PBS containing 0.05% Tween-20) and then blocked for 2 h at room temperature (RT) with blocking buffer (PBS-T and 3% nonfat milk). Serum samples and purified mAbs (Cat. #40150-D001; Sino Biological) were diluted in dilution buffer (PBST and 1% nonfat milk), and 100 µl of each sample was applied to a coated ELISA plate and incubated for 2 h at RT. The plates were then washed and incubated with HRP-labeled anti-human IgG (Cat. #109-035-088; Jackson ImmunoResearch) diluted to 1:3000 in dilution buffer. After incubation for another 1 h at RT, the plates were washed and developed with tetramethylbenzidine (TMB) substrate and quenched with 1 M H2SO4. The sample optical density (OD) was measured by a spectrophotometer at 450 nm. Negative and positive controls were run each time the assay was performed. To ensure the accuracy of the test results, three replicate wells were set up for each sample and the average was taken as the reading of the sample.
2.4 Statistical analyses
Statistical analyses were performed using R (version 4.1.2). The demographic characteristics of infants and mothers were analyzed by descriptive statistics. The Kruskal‒Wallis test was used for the overall statistical analysis between groups, and differences in the level of SARS-CoV-2 IgG antibodies between infants from mothers with and without the COVID-19 vaccine were assessed by the Mann‒Whitney U test. Correlation between the level of SARS-CoV-2 IgG antibodies, infant ages, and days from late vaccine dose to pregnancy was determined by the Spearman test. Significance was defined as a two-sided p < 0.05.
3 RESULTS
3.1 Participants and vaccination characteristics
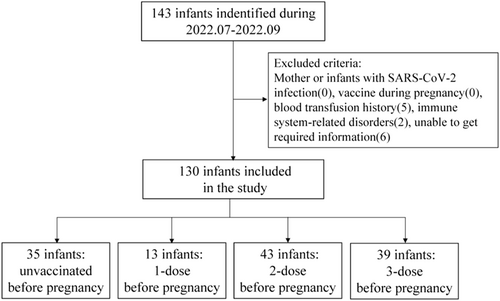
A total of 143 infants with no history of SARS-CoV-2 exposure or infection, 13 were excluded due to blood transfusion, inadequate information, or immune-related disease. Blood samples of 130 infants from 127 mothers were ultimately collected in the study. The study flow chart is shown in Figure 1. The median age of the infants was 26 days (interquartile range [IQR]: [7, 53]) at the time of blood withdrawal during the hospital. The time interval of mothers between administration of the last dose and pregnancy was 65 days (IQR: [29, 99]). CoronaVac (Sinovac life sciences, Co., Ltd.) and COVILO (Beijing Institute of Biological Products Co., Ltd.) inactivated COVID-19 vaccines were the most used. Total serum IgG values were higher in the infants from mothers with 3-dose COVID-19 vaccines before pregnancy than who from mothers did not receive and received 1-dose (19.1 [16.8, 21.0] vs. 15.7 [15.4, 17.1], p = 0.008; 19.1 (16.8, 21.0) vs. 16.6 [16.1, 16.7], p = 0.04). The baseline characteristics of the participants are described in Table 1.
| Maternal vaccine status before pregnancy | ||||
|---|---|---|---|---|
| Characteristics | Unvaccinated (N = 35) | 1-dose (N = 13) | 2-dose (N = 43) | 3-dose (N = 39) |
| Mother | ||||
| Vaccine type | - | Inactivated virus | Inactivated virus | Inactivated virus |
| Age median (IQR)-year | 30 (28, 34) | 28 (25, 31) | 28 (27, 32) | 31 (28, 34) |
| Days from last dose to pregnancy, median (IQR) | - | 57 (8, 66) | 74 (43, 107) | 59 (2, 89) |
| Infant | ||||
| Gestational week, median (IQR) | 38 (36.3, 39.4) | 38.4 (35.7, 39.0) | 38.7 (35.8, 39.4) | 36.9 (33.6, 39.0) |
| Age median (IQR)-day | 33 (5, 62) | 41 (14, 47) | 25 (7, 44) | 20 (7, 59) |
| Female sex, no (%) | 15(42.9%) | 9(69%) | 17(39.5%) | 21(53.8%) |
| SARS-CoV-2 IgG, median (IQR)-optical density (OD450) | 0.032 (0.015, 0.061) | 0.102 (0.068, 0.14) | 0.22 (0.17, 0.39) | 0.885 (0.56, 1.3) |
| Total IgG, median (IQR)-ng/ml | 15.7 (15.4, 17.1) | 16.6 (16.1, 16.7) | 17.4 (16.5, 18.4) | 19.1 (16.8, 21.0) |
- Abbreviations: IQR, interquartile range; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
3.2 SARS-CoV-2 IgG antibodies in infants
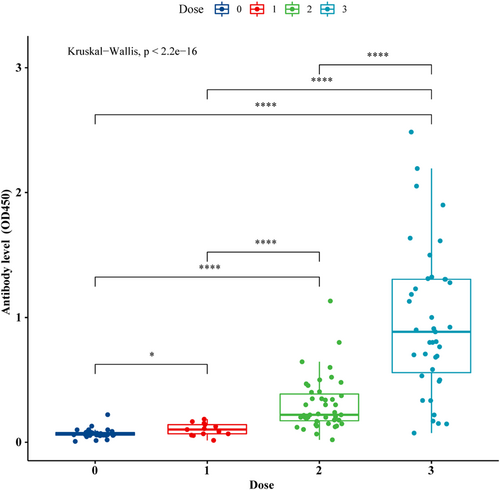
The levels of SARS-CoV-2 IgG antibodies in infants from maternal immunization with 3-dose vaccine (2-dose with additional booster) were significantly highest compared with the 1- and 2-dose groups, median (IQR) of optical density OD450: 0.885 (0.56, 1.3) versus 0.102 (0.068, 0.14) versus 0.22 (0.17, 0.39), p < 0.0001. Furthermore, infants born to mothers with only 1-dose COVID-19 vaccine received before pregnancy also had a higher level of SARS-CoV-2 IgG antibodies than those born to unvaccinated mothers, with median (IQR): 0.102 (0.068, 0.14) versus 0.032 (0.015, 0.061), p < 0.05. (Figure 2).
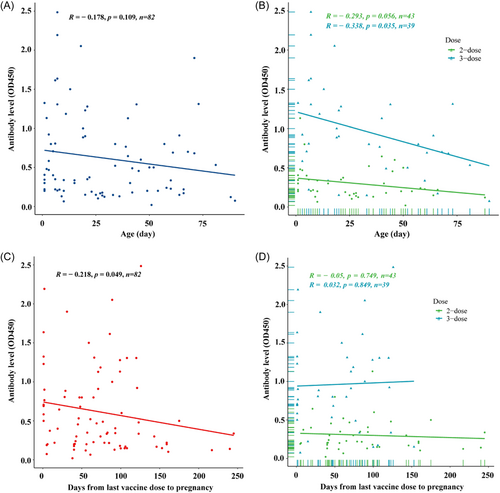
Among infants from mothers with the 3-dose COVID-19 vaccine before pregnancy, the antibody level decreased significantly with age (r = −0.338, p = 0.035), and it was still higher than that in infants from mothers with the 2-dose COVID-19 vaccine (Figure 3A,B). Although the levels of SARS-CoV-2 IgG antibodies was a negative correlation with the time interval from the maternal last vaccine dose to pregnancy (r = −0.218, p = 0.049), the passively acquired antibody was more stable in the infants from mothers with the 3-dose than 2-dose vaccine before pregnancy (r = −0.05, p = 0.749; r = 0.032, p = 0.849; Figure 3C,D).
4 DISCUSSION
These serological data demonstrated that the maternal SARS-CoV-2 IgG antibodies produced from inactivated COVID-19 vaccine before pregnancy can be transferred to newborns by the placenta. The level of SARS-CoV-2 antibodies in infants was positively correlated with the number of COVID-19 vaccine doses mothers received before pregnancy, and a booster dose induced a more robust increase in protective titers for infants.
The goal of maternal immunization, whether before or during pregnancy, is to provide maximal maternal and infant protection. Numerous studies have proven the possibility of transplacental transfer of SARS-CoV-2-specific antibodies that might provide protection to infants.4, 19-22 However, they included persons immunized with the messenger RNA (mRNA) COVID-19 vaccine during pregnancy. Inactivated vaccines were widely used in the general (nonpregnant) population.16, 23 Thus, understanding the effect of the COVID-19 vaccine before pregnancy would be important. Our study supplements existing work by analyzing the SARS-CoV-2 antibody levels in infants from mothers vaccinated before pregnancy.
The impact of immunization timing and vaccine dose on SARS-CoV-2 transplacental antibody transfer may influence neonatal seroprotection. According to previous studies, the COVID-19 vaccine of pregnant women during the second trimester could be effective to achieve maternal protection and newborn safety during the pandemic.21, 24, 25 In this study, we have found that the levels of SARS-CoV-2 antibodies were higher in infants from mothers immunizing the COVID-19 vaccine than unvaccinated before pregnancy. Although the level of SARS-CoV-2 IgG antibody decreased with the age of infants, significantly higher SARS-CoV-2 IgG antibodies levels in infants from the maternal 3-dose of inactivated COVID-19 vaccine before pregnancy compared with the 2-dose. Maternal transferred antibodies are an important protection against infection.26, 27 Those results suggest that maternal immunization before pregnancy could be helpful for infants, and a booster vaccination may provide better and longer COVID-19 protection for both mothers and infants during pregnancy and after birth.
This study has several limitations. It was conducted in a single center and a single time point of serum antibody level, and limited sample volume was detected for each infant due to obvious ethical reasons. Second, the maternal SARS-CoV-2 IgG antibodies were not evaluated during pregnancy. Although the level of SARS-CoV-2 IgG antibodies would be significantly higher after the third (booster) dose compared with the primary 2-dose vaccine series, there is uncertainty as to the passive transmission ratio of maternal antibodies to infants.
Given the increased risk for severe disease with systematic SARS-CoV-2 infection in the two special populations of pregnant women and infants, maternal immunization would be significant because no COVID-19 vaccines have been approved for infants under 6 months of age. Therefore, three doses of COVID-19 vaccination could be recommended for women who trying to get pregnant now or might become pregnant in the future, which may protect both mothers and infants from SARS-CoV-2 infection. The protective effects and dynamics change of passive transfer of SARS-CoV-2 antibodies acquired by mothers before pregnancy passively transfer to their infants may require further analysis.
ACKNOWLEDGMENT
This study was supported by the National Clinical Research Center for Child Health and Disorders General Project (No. NCRCCHD-2019-GP-04) and Youth Project (No. NCRCCHD-2021-YP-03); Chongqing Talent Program (CQYC20210303393); Program for Youth Innovation in Future Medicine, Chongqing Medical University.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




