Characterization and phylogenetic analysis of a neurovirulent Zika virus isolated from Cambodia in 2019
Yi-Fei Zhang, Jing-Jing Guo, and Fan Yang contributed equally to this study.
Abstract
The geographic range of Zika virus (ZIKV) has expanded from Asia to the Americas, leading to the 2015–2016 pandemic with enhanced neurovirulence. At present, ZIKV is continuously circulating in many Southeast Asian countries. Unfortunately, the persistent evolution of ZIKV in Southeast Asia and its influence on the biological characteristics of the virus remain incompletely understood. In this study, the in vitro and in vivo properties of a new ZIKV isolate obtained from Cambodia in 2019 (CAM/2019) were characterized and compared with those of the Cambodian strain (CAM/2010). Compared with CAM/2010, the CAM/2019 virus showed similar plaque morphology and growth curves in cell cultures and induced comparable viremia and organ viral loads profiles in both BALB/c and A129 (IFNAR1−/−) mice upon intraperitoneal (i.p.) inoculation. Remarkably, the CAM/2019 virus exhibited enhanced neurovirulence in neonatal mice compared with CAM/2010, with a 74-fold reduction in the 50% lethal dose (LD50). Consistently, CAM/2019 produced higher viral loads in the brains of BALB/c neonatal mice than CAM/2010 did. Sequence alignment showed that the CAM/2019 virus has acquired 12 amino acid substitutions, several of which were found to be associated with neurovirulence. In particular, the CAM/2019 virus shared an A1204T substitution in NS2A with the Thai isolate SI-BKK02 that was isolated from a microcephaly case. Taken together, our results indicate that a ZIKV strain isolated with specific mutations has emerged in Cambodia, highlighting the need for extensive molecular and disease surveillance in Cambodia and other Asian countries.
1 INTRODUCTION
Zika virus (ZIKV), which belongs to the genus Flavivirus, is a single-stranded, positive-sense enveloped RNA virus with a genome of approximately 10.8 kb.1 The genome of ZIKV encodes three structural proteins, namely, the capsid (C), premembrane/membrane (prM), and envelope (E) proteins, and seven nonstructural proteins, namely, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5.2 The structural proteins, together with genomic RNA, are components of virions. Nonstructural proteins participate in viral RNA replication, virion assembly, and evasion of the host immune response.3 ZIKV is mainly transmitted to humans through the bites of infected mosquitoes in many tropical and subtropical regions. In general, ZIKV infection is thought to cause mild symptoms, such as fever, fatigue, rashes, and arthritis. However, recent evidence has clearly demonstrated that ZIKV infection can lead to more serious consequences, such as Guillain‒Barre syndrome in adults and microcephaly in neonates.1, 4, 5
Phylogenetic analysis showed that ZIKV can be divided into African and Asian lineages. In 1966, the prototypic strain of the ZIKV Asian lineage was first isolated from mosquitoes in Malaysia.6 The first recorded outbreak of ZIKV in Asia may have occurred in central Java, Indonesia, in 1977.7 In 2007, another outbreak of ZIKV was reported on the Yap Islands, Federated States of Micronesia. Whole-genome sequencing indicated that the outbreak was caused by the introduction of an Asian ZIKV lineage.8 Subsequently, there was a large outbreak in French Polynesia in the South Pacific in 2013, and ZIKV was first linked to Guillain‒Barre syndrome in this outbreak.9-11 The ZIKV isolates from French Polynesia were similar to those from the Yap Islands in 2007 and Cambodia in 2010, and both strains probably originated from Southeast Asia.12 Subsequently, ZIKV spread to the Americas, and a major outbreak of ZIKV disease began in Brazil and then spread to other Latin American countries in 2015.11 In the Brazilian outbreak, ZIKV infection was unexpectedly linked to microcephaly in newborns.13 Notably, between 2007 and 2016, several Southeast and South Asian countries, such as Cambodia,14, 15 India,16 Indonesia,17 Philippines,18 Singapore,19 Japan,20 Thailand,21 and Vietnam,22-25 also reported ZIKV outbreaks and sporadic indigenous transmission events. At present, the continuous circulation and evolution of ZIKV remains a public health problem in Southeast Asia.
The rapid geographic expansion and increase in severe clinical outcomes were largely attributed to the molecular evolution of ZIKV. For example, the T106A substitution in the C protein facilitated transmission by the mosquito vector, as well as infection in both human cells and immunodeficient mice.26 The S139N substitution in the prM protein significantly increased the neurovirulence of ZIKV and the number of microcephaly cases.27 The V473M substitution in the E protein increased neurovirulence, maternal-to-fetal transmission, and viremia to facilitate urban transmission.28 In addition, the A982V substitution in the NS1 protein increased NS1 antigenemia to improve ZIKV infectivity and prevalence in mosquitoes.29 Due to the lack of an extensive surveillance system for ZIKV in Southeast Asia, the genomic and biological properties of ZIKV isolates in Southeast Asian countries have not been thoroughly investigated.
In this study, we characterized the in vitro and in vivo phenotypes of a new isolate of ZIKV from Cambodia in 2019 (CAM/2019) using the Cambodian isolate FSS13025 as a reference strain (CAM/2010). Interestingly, the new Cambodian isolate showed enhanced neurovirulence and contained several critical amino acid mutations throughout the genome.
2 MATERIALS AND METHODS
2.1 Cells and viruses
The baby hamster kidney fibroblast cell line BHK-21 (ATCC, CCL10), Aedes albopictus cell line C6/36 (ATCC, CRL-1660), and human neuroblastoma cell line SH-SY5Y (ATCC CRL-2266) were cultured using DMEM (Thermo Fisher Scientific), RPMI 1640 (Thermo Fisher Scientific) and DMEM/F12 (Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific), respectively.
The ZIKV strain CAM/2019 was isolated from a saliva sample from a 33-year-old Chinese male patient returning from Cambodia in December 2019. The whole genome of CAM/2019 was deposited in GenBank under accession number ON209935. The ZIKV strain FSS13025 (CAM/2010) was originally isolated from a Cambodian patient in 2010. All viral stocks were prepared in C6/36 cells and titrated by a standard plaque formation assay.
2.2 Plaque assay
The virus samples were serially diluted 10-fold with DMEM containing 2% FBS, and 400 μl of each diluent was added to 12-well plates covered with BHK-21 cells; the plates were then incubated at 37°C with 5% CO2 for 1 h. The supernatants containing the viruses were aspirated, and 1 ml of DMEM containing 1% low-melting-point agarose (Promega) and 2% FBS was added to each well. The infected cells were cultured for another 4 days. The cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet solution. Plaques were counted for calculation of the viral titers.
2.3 Growth kinetics
BHK-21, C6/36, and SH-SY5Y cells were transferred to 24-well plates and cultured at 37°C with 5% CO2. Viral infection was carried out when the cell density was approximately 90%. The cell supernatant was discarded, and culture medium containing virus at MOI = 0.1 was added to the different cell lines, which were then incubated at 37°C for 1 h. Then, the supernatant was discarded, and 0.5 ml of fresh medium was added to each well. Infected cells were cultured at 37°C with 5% CO2, and the cell supernatant was collected at 0, 24, 48, and 72 h after changing the solution. Viral RNA loads in the cell supernatant were determined by plaque and RT-qPCR assays, and the growth curves of the virus with different cells were plotted by GraphPad Prism 8 software.
2.4 Immunofluorescent assay
The immunofluorescence assay (IFA) was performed to detect viral protein expression. BHK-21 cells were infected at MOI = 0.1, and the supernatant was discarded at 24, 48, and 72 h. The cells were fixed with methanol/acetone (v/v: 7/3) at −20°C and incubated with the anti-Zika ENV mAb (1:1000 diluted, BioFront Technologies) at 37°C for 1 h. The cells were then incubated with goat anti-mouse IgG-Alexa Fluor 488 (1:200 diluted, Gene‒Protein Link) at 37°C for 1 h. For cell nucleus staining, 4,6-diamidino-2-phenylindole (DAPI, 0.5 ng/μl) was added to the wells, and the cells were incubated for 5 min. An Olympus IX73 microscope was used for image acquisition.
2.5 Neurovirulence in neonatal mice
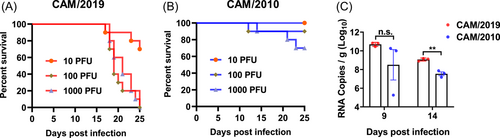
For the mouse neurovirulence test, 1-day-old neonatal BALB/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Sixty mice were randomly divided into six groups (n = 10 per group). The CAM/2019 and CAM/2010 strains were intracerebrally (i.c.) injected with 10, 100, or 1000 PFU. The mice were observed, and clinical signs were recorded daily for 25 days. Survival analysis was performed using GraphPad Prism 8 software, and the LD50 (lethal dose, 50%) was calculated by using the Reed–Muench method. At 9 and 14 days post-infection (dpi), the viral loads in the brains of 1-day-old neonatal BALB/c mice challenged with 100 PFU of the CAM/2019 and CAM/2010 viruses were harvested, weighed, homogenized, and analyzed by RT-qPCR. The viral loads are expressed as RNA copies per gram.
2.6 Virulence in BALB/c and A129 (IFNAR1−/−) mice
To test virulence in adult mice, immunocompetent BALB/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. A129 (IFNAR1−/−) mice lacking the type I interferon receptor were bred in our laboratory. Briefly, 6-week-old BALB/c and A129 (IFNAR1−/−) mice were infected with 1 × 105 PFU of the CAM/2019 and CAM/2010 viruses via the intraperitoneal (i.p.) route (n = 3 per group). At 1, 2, 3, 5, and 7 dpi, blood was collected and analyzed by RT-qPCR to measure viremia. The viral loads are expressed as RNA copies per milliliter. At 3 dpi, the various indicated tissues of A129 mice were harvested, weighed, homogenized, and analyzed by RT-qPCR to determine the tissue distribution. The viral loads are expressed as RNA copies per gram.
2.7 ZIKV RNA quantification by RT-qPCR
Viral RNA was extracted using a Purelink RNA Mini Kit (Thermo Fisher Scientific) and eluted in 50 μl of RNase-free water. A specific probe (5′-AGCCTACCTTGACAAGCAATCAGACACTCAA-3′) and primer set (ZIKV_1193F: 5′-CCGCTGCCCAACACAAG-3′; ZIKV_1269R: 5′-CCACTAACGTTCTTTTGCAGACAT-3′) were used to determine the number of ZIKV RNA copies.28 RT-qPCR was performed using the One-Step PrimeScript™ RT-PCR kit (TaKaRa) and detected using the LightCycler® 480 system (Roche).
2.8 ZIKV genome sequencing
A total of 15 primer pairs (Supporting Information: Table S1)30 were used to generate overlapping amplicons spanning the entire coding sequence (CDS) using the SuperScript III One-Step RT-PCR System (Thermo Fisher Scientific). The PCR products were then sequenced by an ABI 3730 Sanger sequencing-based genetic analyzer.
2.9 Sequence alignment and phylogenetic analysis
Sequence alignment was performed to analyze the nucleotide and amino acid sequence consistency of each protein of the CAM/2019 and CAM/2010 viruses with DNAMAN software. To construct a phylogenetic tree, 28 representative Asian lineages of ZIKV were aligned using MAFFT version 7.31 The phylogenetic tree was then estimated by using the maximum-likelihood method available within the IQ-TREE program under the best-fit substitution model,32, 33 with ultrafast bootstrap support values calculated from 1000 replicate trees. Bootstrap values higher than 70% were considered significant.
2.10 Statistical analysis
All data were analyzed with GraphPad Prism 8.4.0 software. Unless specified, data are presented as the mean ± SEM in all experiments. The statistical significance was assessed by an unpaired t-test and two-way ANOVA. In the survival test, the statistical significance was analyzed by Wilcoxon log-rank (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; n.s., not significant).
3 RESULTS
3.1 CAM/2019 showed similar in vitro replication properties as CAM/2010
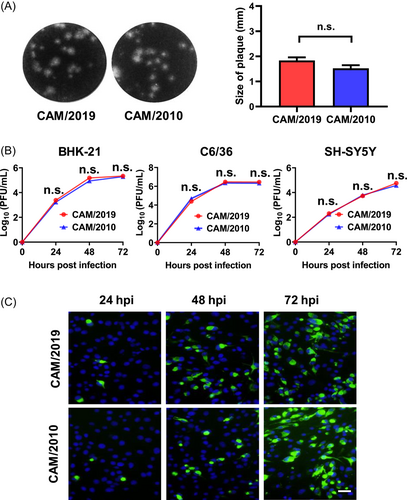
The Cambodian strain CAM/2010 is a good representative of the prepandemic ZIKV isolate with less neurovirulence and reduced risk of inducing microcephaly.27 In the present study, we first compared the in vitro phenotypes of CAM/2019 and CAM/2010 viruses in three ZIKV-susceptible cell lines (BHK-21, C6/36, and SH-SY5Y cells). CAM/2019 infection produced typical homogeneous small plaques with an average diameter of 1.837 mm in BHK-21 cells, similar to the phenotype of CAM/2010, with an average diameter of 1.523 mm (Figure 1A). Then, the replication characteristics of the CAM/2019 and CAM/2010 viruses in BHK-21, C6/36, and SH-SY5Y cells were determined by using plaque assays and RT‒qPCR assays. The plaque results showed that CAM/2019 and CAM/2010 infection exhibited similar replication kinetics, and the peak titers reached 105–6, 106–7, and 104–5 PFU/ml for BHK-21, C6/36, and SH-SY5Y, respectively (Figure 1B). The RT‒qPCR results were also consistent with the plaque assay results (Supporting Information: Figure S1). In addition, the IFA results also showed that ZIKV E protein-specific proteins were detected in both CAM/2019- and CAM/2010-infected BHK-21 cells in a time-dependent manner (Figure 1C).
3.2 CAM/2019 showed enhanced neurovirulence in neonatal mice compared with CAM/2010
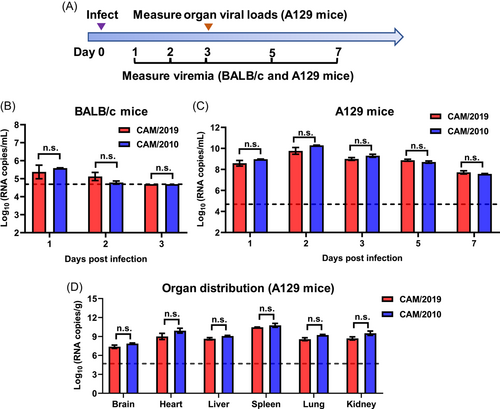
First, we characterized the in vivo infectivity of CAM/2019 in two well-established BALB/c and A129 mouse models34 (Figure 2A) in comparison with that of CAM/2010. Following i.p. inoculation, transient viremia was detected in BALB/c mice infected with the CAM/2010 and CAM/2019 viruses, peaking at 1 dpi and decreasing to below the detection limit at 3 dpi (Figure 2B). Similarly, CAM/2010 and CAM/2019 infection resulted in continuous viremia in A129 mice from days 1 to 7, with peak titers of 109–1010 RNA copies/ml at 2 dpi (Figure 2C). At 3 dpi, infectious ZIKV could be detected in all the indicated organs (Figure 2D). These results suggested that the CAM/2010 and CAM/2019 viruses exhibited similar viral titers in blood and multiple organs without statistically significant differences.
Then, we further characterized the in vivo neurovirulence of the CAM/2019 virus in the 1-day-old neonatal BALB/c mouse model in comparison with that of CAM/2010. The results showed that all mice challenged with 1000 and 100 PFU of CAM/2019 succumbed within 25 days, and 10 PFU of CAM/2019 also caused death in 30% of neonatal mice (Figure 3A). However, the survival rates were 100%, 90%, and 70% in the three dose groups (10, 100, and 1000 PFU of CAM/2010), respectively (Figure 3B). The LD50 of CAM/2019 (22 PFU) was 74-fold lower than that of CAM/2010 (1637 PFU). In particular, all the dead mice exhibited typical nervous system symptoms, such as reduced movement, motor weakness, and bilateral hind-limb paralysis. We also determined the viral loads in the brains of 1-day-old neonatal BALB/c mice challenged with 100 PFU of the CAM/2019 and CAM/2010 viruses at 9 and 14 dpi by an RT-qPCR assay. The results showed that CAM/2019 infection was significantly higher than CAM/2010 infection at 14 dpi (Figure 3C). Taken together, these results clearly demonstrate that CAM/2019 virus had an enhanced neurovirulence phenotype in neonatal mice compared with CAM/2010.
3.3 CAM/2019 harbored critical amino acid mutations related to neurovirulence
Furthermore, to obtain the whole-genome sequence of the CAM/2019 virus, a total of 15 primer pairs (Supporting Information: Table S1) were used to generate overlapping amplicons spanning the entire coding sequence. The resulting PCR products had an average length of 800 base pairs (Figure 4A). Then, the PCR products were sequenced by an ABI 3730 Sanger-based genetic analyzer and assembled using DNAMAN software. The partial untranslated regions (UTRs) and complete CDS of the CAM/2019 strain were submitted to GenBank under accession no. ON209935. The complete CDS of the CAM/2019 strain was 10 272 nucleotides (nt) in length, and the only open reading frame (ORF) encoded 3424 amino acids (aa). DNAMAN was used to analyze the nucleotide and amino acid sequence consistency of each protein of the CAM/2019 and CAM/2010 viruses. The result is shown in Figure 4B. The ORF encodes a polyprotein consisting of three structural proteins, namely, C (366 nt/98.36% or 122 aa/99.18%), prM (504 nt/98.61% or 168 aa/98.21%), and E (1512 nt/98.08% or 504 aa/99.80%), and seven nonstructural proteins, namely, NS1 (1056 nt/97.73% or 352 aa/99.72%), NS2A (678 nt/97.94% or 226 aa/99.12%), NS2B (390 nt/97.44% or 130 aa/98.46%), NS3 (1851 nt/98.54% or 617 aa/100%), NS4A (381 nt/96.85% or 127 aa/100%), NS4B (822 nt/98.05% or 274 aa/100%), and NS5 (2712 nt/98.01% or 904 aa/99.67%). A total of 12 amino acid mutations were identified between the CAM/2019 and CAM/2010 viruses, including C-T106A, prM-V123A, prM-N130S, prM-M151L, E-V763M, NS1-A982V, NS2A-A1204T, NS2A-P1274L, NS2B-A1477T, NS5-V2878A, NS5-M3392V, and NS5-V3403M (Figure 4C).

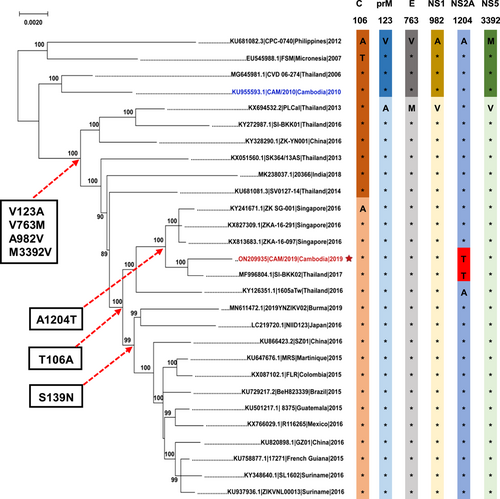
Finally, we mapped these amino acid substitutions on a maximum-likelihood tree and sought to identify the genetic determinants associated with increased neurovirulence of the CAM/2019 strain. Phylogenetic tree analysis showed that the CAM/2019 strain belonged to the Asian lineage and was most closely related to the ZIKV strain SI-BKK02 (accession number MF996804.1) from Thailand in 2017, with 99.63% nucleotide and 99.91% amino acid similarity. As previously described, the American isolates obtained during the pandemic contained the unique S139N mutation. Compared with the prepandemic isolates (2007–2012), including the CAM/2010 strain, most recent isolates from Southeast Asia (2013–present) have subsequently acquired a panel of critical mutations, including T106A, V123A, V763M, A982V, and M3392V. Interestingly, a special A1204T mutation in the NS2A protein appeared in both CAM/2019 and SI-BKK02 (Figure 5). As the SI-BKK02 strain was isolated from a microcephaly case, the biological relevance of the association between the A1204T mutation and neurovirulence and microcephaly demands further investigation.
4 DISCUSSION
In this study, we comprehensively characterized a newly isolated ZIKV strain from Cambodia in CAM/2019 in comparison with a historical isolate CAM/2010. There was no significant difference between CAM/2019 and CAM/2010 in growth curves in BHK-21, C6/36, and SH-SY5Y cells, as well as viremia and organ viral loads in adult A129 and BALB/c mouse models. Remarkably, CAM/2019 infection exhibited enhanced (74-fold) neurovirulence in comparison with CAM/2010 in neonatal mice, and the LD50 of CAM/2019 was calculated to be 22 PFU. In addition, higher viral loads were detected in the brains of CAM/2019-infected BALB/c neonatal mice than CAM/2010-infected animals at 14 dpi. Previously, Yuan et al. have shown that 10 PFU of GZ01 and SZ01 strains (GenBank numbers KU820898 and KU866423) caused 100% death in 1-day-old neonatal mice.27 Further comparison of mouse neurovirulence of CAM/2019 and other American strains (such as GZ01 and SZ01) would help characterize the virulence phenotype of these isolates with different genetic backgrounds.
Phylogenetic analysis further indicated that six amino acid mutations were perhaps associated with increased neurovirulence of the CAM/2019 virus. Among the six mutations, T106A in the C protein could directly increase infectivity and improve packaging efficiency as well as promote the transmissibility of the virus in mammalian hosts and mosquito vectors, thus greatly increasing the epidemic potential of ZIKV during its transmission cycle.26 A982V in NS1, which contributes to increasing the secretion of NS1 and enhancing the uptake of the virus by mosquitoes, thus significantly improving the transmission efficiency of ZIKV to mosquitoes; the NS1 protein also inhibited the induction of interferon-β.29, 35, 36 Shan et al. reported that V763M in the E protein significantly enhanced neonatal mouse neurovirulence and mother-to-child transmission during pregnancy in mice. M3392V in the NS5 protein increases the death of neonatal mice, while V123A in prM reduces the death of neonatal mice.28 Notably, we found that the CAM/2019 and SI-BKK02 strains both exhibited a novel A1204T mutation in the NS2A protein that was different from those in other Asian ZIKV strains.37 The flavivirus NS2A protein is involved in viral RNA synthesis,38 virus assembly,39 membrane rearrangement, and immunomodulation of the innate immune response.3 Moreover, a previous study indicated that the A117V mutation in the NS2A protein influences the virulence of ZIKV in mice.40 However, the potential role of the A1204T mutation in the neurovirulence of CAM/2019 in neonatal mice remains unknown. In future work, we will use reverse genetics to identify whether this site mutation is responsible for the increased neurovirulence of CAM/2019.
ZIKV has been circulating in Southeast Asia for at least 50 years, and until now, its spread in the region has been underestimated. Although two Zika-related fetal microcephaly cases in Thailand and a case in Vietnam have been reported,24, 37, 41 the key amino acid sites of ZIKV that cause microcephaly in Southeast Asia have not been identified. Therefore, our present study not only provides a scientific basis for the evolution of ZIKV in Southeast Asia but also highlights the significance of continued surveillance during future ZIKV outbreaks.
AUTHOR CONTRIBUTIONS
Cheng-Feng Qin and Yong-Qiang Deng conceived, designed, and supervised the study. Yi-Fei Zhang, Jing-Jing Guo, and Fan Yang performed a majority of the experiments and analyzed the data. Hang-Yu Zhou, Na-Na Zhang, Xiao-Chuan Xiong, and Yue Feng contributed to experiments and data analysis. All authors have read and approved the contents of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (NSFC no. 31770190) and the Shenzhen Science and Technology Plan Project (JCYJ20190807103005636). C.F.Q. was supported by the National Science Fund for Distinguished Young Scholars (81925025), the Innovative Research Group (81621005) from the NSFC, and the Innovation Fund for Medical Sciences (2019-I2M-5-049) from the Chinese Academy of Medical Sciences.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
All animal experiments were performed in strict accordance with the guidelines of the Chinese Regulations of Laboratory Animals (Ministry of Science and Technology of the People's Republic of China) and Laboratory Animal-Requirements of Environment and Housing Facilities (GB 14925-2010, National Laboratory Animal Standardization Technical Committee). All procedures were approved by the Animal Experiment Committee of Laboratory Animal Center, AMMS, China (IACUC-IME-2021-010).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




