Discriminating endogenous vaccine-elicited anti-Spike antibody responses from exogenous anti-Spike monoclonal antibodies: The case of Evusheld
Immunocompromised patients represent the most problematic cohort of coronavirus disease 2019 (COVID-19) management nowadays, given the inability of most to mount a protective antibody response after vaccination. With the current Spike-based vaccines, most of them are actually unable to mount detectable anti-Spike immunoglobulin G (IgG) levels and are hence repeatedly boosted with multiple vaccine doses, hoping for a rescue. Since Spring 2022, most immunocompromised patients in many western countries are under pre-exposure prophylaxis with Evusheld™. Evusheld™ is a cocktail of two extended half-life anti-Spike monoclonal antibodies (mAbs), namely, tixagevimab and cilgavimab, which, once administered intramuscularly or intravenously at the recommended dosage of 300 + 300 mg, produce stable anti-Spike IgG serum levels for at least 6 months, which are 10–100-folds higher than those seen after COVID-19 boosts. Current guidelines recommend a repeat dose every 6 months.1 This makes it impossible to assess a vaccine-elicited endogenous anti-Spike antibody response on the background of Evusheld™. While it remains possible to discriminate on the basis of cell-mediated immune responses (induced by vaccination but not by Evusheld™), cell-based assays are not widely available, are not high-throughput, and to date, anti-Spike-neutralizing antibodies are the best surrogate for vaccine efficacy.2 There is hence an urgent need for serological assays that can discriminate endogenous anti-Spike IgG from Evusheld™ to avoid unnecessary boosts and to stop unnecessary pre-exposure prophylaxis. In the absence of such a test, we are likely dealing with a never-ending cycle of boosts and Evusheld™ doses that will never generate evidence to stop one of the two. The easiest way to develop such an assay is to rely on Spike versions that we know are fully resistant to neutralization by both Evusheld™ ingredients. The individual Spike mutations that provide full resistance to both ingredients of Evusheld™ are nowadays fully known (i.e., R346I or K444E/Q/R for cilgavimab [>200-fold reduction], and F486S [>600-fold reduction] for tixagevimab),3 so that a synthetic Spike antigen with such a minimum set of mutated amino acid residues could preserve binding to vaccine-elicited polyclonal IgGs but not to Evusheld™.
Another point to consider is how prophylactic, exogenous anti-Spike mAbs (eventually administered repeatedly to preserve very high serum levels, such as is the case for Evusheld™) could prevent the elicitation of an active endogenous immune response against the Spike protein encoded by or included within COVID-19 vaccines. Both cilgavimab and tixagevimab achieve very high serum levels and tissue penetration throughout the body,4 likely including the site of vaccine injection, which could theoretically neutralize the totality of the vaccinal Spike antigen, preventing its presentation to the host immune system. The emergency use authorization granted by Food and Drug Administration to Evusheld™ currently contraindicates Evusheld™ within 15 days since vaccination, likely to better impute eventual adverse events5: clearly, this recommendation conflicts with the recommended repeated vaccine schedule unless intervals are introduced between Evusheld™ doses to allow booster administrations. While Evusheld™ has not been specifically investigated for this risk yet, we can gather two levels of evidence, namely, the impact of other anti-Spike mAb therapies on the endogenous anti-Spike response after natural infection alone (Figure 1A) or natural infection followed by vaccination (Figure 1B).
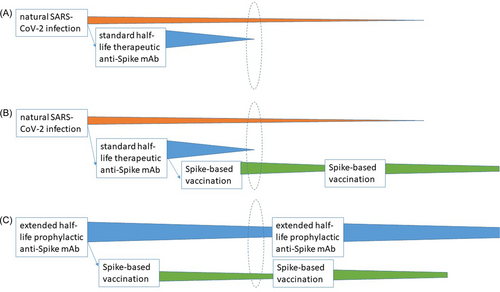
The first type of evidence is unlikely to be informative, given that the mAb dose given therapeutically is unable to neutralize the entire amount of Spike proteins within the human body, leaving the rest able to elicit an endogenous immune response (Figure 1A). Accordingly, Zhang et al.6 actually demonstrated 1.4- to 4.1-fold higher anti-Spike IgG levels after natural severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at Days 15–85 in BLAZE-1 trial patients who received bamlanivimab alone (700 mg [n = 100], 2800 mg [n = 106], or 7000 mg [n = 98]), or bamlanivimab (2800 mg) and etesevimab (2800 mg) together (n = 111) compared to those who received placebo (n = 153). Nevertheless, there seems to be an impact on IgM elicitation: Kim et al.7 reported that, in a retrospective cohort study of Veterans Health Administration, 64 patients treated with bamlanivimab, bamlanivimab + etesevimab, or casirivimab + imdevimab showed 85%–90% reduced anti-Spike IgM response since the seroconversion stage (10–19 days) until the maximum antibody stage (20–39 days) compared to 34 untreated cases.
The second line of evidence needs to take into account the relative contribution of postinfectious immunity to vaccine-elicited immunity, and the time between anti-Spike mAb administration and vaccine administration: the latter parameter is dispensable for Evusheld™ (given the long half-life and the repeated dosing), but is instead fundamental to interpret the impact of other anti-Spike IgG mAbs having serum half-lives around 20 days. Benschop et al.8 demonstrated that at a median of 67 days after bamlanivimab, 167 healthcare workers participating in ACTIV-2 RCT had minimally reduced anti-Spike responses to messenger RNA vaccines, but at that time there was minimal bamlanivimab circulating in the serum. Infusion of current IVIg preparations that contain anti-SARS-CoV-2 IgG at least 2 weeks after vaccination does not significantly alter serum anti-SARS-CoV-2 IgG responses.9 The only informative study to date is a case report by Schultz-Cherry et al.,10 who found that an individual who received two BNT162b2 doses 16 and 37 days after bamlanivimab therapy for symptomatic COVID-19 had similar anti-receptor-binding domain (RBD) IgGs as other untreated convalescents vaccinated or not with BNT162b2, which persisted for at least 80 days: the authors separated bamlanivimab from vaccine-elicited antibodies using enzyme-lined immunoassay relying on RBDs from bamlanivimab-resistant SARS-CoV-2 variants of concerns. No studies have been conducted for casirivimab + imdevimab, sotrovimab, or Evusheld™.
Additional hints come from experience with different viruses and passive immunotherapies. It has been previously shown that after high-dose intravenous Ig (>10 mg/kg) antibody responses to the measles vaccine were inhibited for up to 5 months and those to the rubella vaccine were inhibited for 2 months.11
In conclusion, while in the coming future COVID-19 vaccines based on antigens other than Spike and robust cell-mediated immunoassays could facilitate the distinction between vaccine-elicited endogenous responses and exogenous mAbs, right now we urgently need the development of discriminatory seroassays, which will pave the way to studies on the inhibitory potential of Evusheld™ during Spike-based vaccine boosts, and finally provide hints to terminate either boosts or mAb prophylaxis (Figure 1C). Such studies are even more needed nowadays as the potential efficacy of Evusheld™ is decreasing.1, 12
AUTHOR CONTRIBUTIONS
Daniele Focosi designed the manuscript, and Shmuel Shoham and Arturo Casadevall revised the draft.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
This manuscript did not generate novel data. Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.




