Postexposure-vaccine-prophylaxis against COVID-19
Israeli MoH Registry Number: HMO-0372-20
Zohar Shmuelian and Yehuda Warszawer contributed equally to this report.
Maruotti, Jona Lasinio, and Galun contributed equally to this article.
Abstract
During the COVID-19 pandemic, postexposure-vaccine-prophylaxis is not a practice. Following exposure, only patient isolation is imposed. Moreover, no therapeutic prevention approach is applied. We asked whether evidence exists for reduced mortality rate following postexposure-vaccine-prophylaxis. To estimate the effectiveness of postexposure-vaccine-prophylaxis, we obtained data from the Israeli Ministry of Health registry. The study population consisted of Israeli residents aged 12 years and older, identified for the first time as PCR-positive for SARS-CoV-2, between December 20th, 2020 (the beginning of the vaccination campaign) and October 7th, 2021. We compared “recently injected” patients—that proved PCR-positive on the same day or on 1 of the 5 consecutive days after first vaccination (representing an unintended postexposure-vaccine-prophylaxis)s—to unvaccinated control group. Among Israeli residents identified PCR-positive for SARS-CoV-2, 11 687 were found positive on the day they received their first vaccine injection (BNT162b2) or on 1 of the 5 days thereafter. In patients over 65 years, 143 deaths occurred among 1412 recently injected (10.13%) compared to 255 deaths among the 1412 unvaccinated (18.06%), odd ratio (OR) 0.51 (95% confidence interval [CI]: 0.41–0.64; p < 0.001). A significant reduction in the death toll was observed among the 55–64 age group, with 8 deaths occurring among the 1320 recently injected (0.61%) compared to 24 deaths among the 1320 unvaccinated control (1.82%), OR 0.33 (95% CI: 0.13–0.76; p = 0.007). Postexposure-vaccine-prophylaxis is effective against death in COVID-19 infection.
1 INTRODUCTION
SARS-CoV-2 vaccination has proved effective in preventing clinical COVID-19 infection.1 Numerous drugs had shown some benefit to patients with COVID-19. These include the following compounds: Remdesivir,2 Paxlovid which received an emergency use in PCR-positive patients,3 the anti-inflammatory drugs Dexamethasone,4 Baricitinib5 and anti-IL6 receptor6 and some compounds in clinical development.7 However, the therapeutic benefit from most of these treatments is still suboptimal so additional therapeutic approaches are needed to reduce the loss of human lives. One potential clinical approach is postexposure vaccination prophylaxis (vaccination after exposure to a pathogen). Postexposure vaccination prophylaxis is an old approach used to attenuate severe infections by boosting an efficient immune response. This is adopted following bacterial infections in the case of tetanus, but is more common following exposure to viral infections. Although the postexposure approach, in many occasions, specific to the viral infection context, as for hepatitis B virus8 or after a bite from a rabies infected dog, the concept is generally the same.9 Thus, active vaccination with the attenuated/killed pathogen or viral associated protein is a very effective mean to attenuate and almost eliminate any infection related symptoms. This approach is now also suggested for Ebola virus infection.10 In accordance, it was recently shown that the overall neutralizing potency of plasma is greater following vaccination compared to natural infection with SARS-CoV-2.11 In the case of vaccinating a patient with COVID-19 respiratory illness, it is expected that upon vaccination, an efficient immune response against SARS-CoV-2 will develop in a remote site from the pneumonitis developed in the lung, possibly preventing respiratory deterioration. Postexposure vaccination prophylaxis is in particular relevant, in light of the waning immunity against COVID-19 after BNT162b2 vaccination12-16 We wish to determine in the real world, whether vaccinating patients who were already infected by SARS-CoV-2 will benefit from postexposure prophylaxis vaccination. This could be in particular relevant as a measure undertaken together with isolation strategies following exposure. We performed this investigation by a data mining study on an available national, subnational (one Israeli Health Management Organization [HMO]) and institutional database.
2 METHODS
2.1 Study design
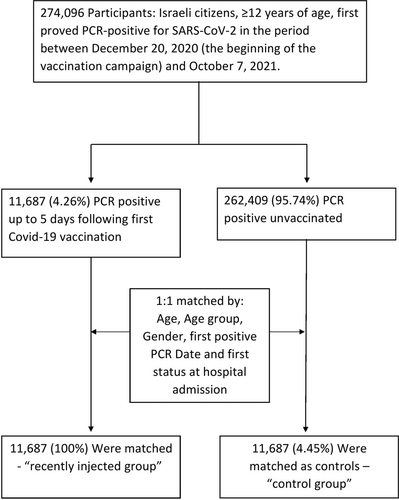
In this observational study, we analyzed nationwide surveillance data obtained from the Israeli Ministry of Health registry to assess the effectiveness of the BNT162b2 vaccine as postexposure prophylaxis approach against the clinical outcomes of SARS-CoV-2 infection. Our study population consisted of Israeli residents aged 12 years and older, identified for the first time as PCR-positive for SARS-CoV-2, between December 20th, 2020 (the beginning of the vaccination campaign) and October 7th, 2021, and did not include patients that were found PCR-positive 6 days or later after they received the first vaccine injection (Figure S1). The data on each patient included: age, gender, first positive PCR date, first vaccination date, hospitalization dates (arrival and discharged), first status at hospital admission and date of death. We compared two groups: (Group 1) the “recently injected” test group—patients proved PCR-positive on the same day or on 1 of the 5 consecutive days after receiving their first vaccine injection. This group was chosen because it practically represents an unintended postexposure prophylaxis treatment. (Group 2) The control unvaccinated group. To establish this control group, we matched the “recently injected” group to the unvaccinated controls using the following variables: gender, age, first status at hospital admission and date of the first positive PCR, variables associated with the severity of COVID-19. To confirm the similarity between the groups, “recently injected” and “unvaccinated,” we also investigated a large HMO database of 500 000 registered individuals (IRB approval number COM1-0167-21) and a cohort of 4500 institutional data (IRB approval number HMO-0372-20) for epidemiological and co-morbidities measures, this data is detailed in Supporting Information Material.
2.2 SARS-CoV-2 strains
Most of the SARS-CoV-2 infection cases in the “recently injected” group, occurred at the beginning of the vaccination campaigns, and can be divided to two groups: (Group 1) from December 20, 2020 until approximately March 31, 2021 including adults from 16 years old; (Group 2) from June 6, 2021 until approximately September 30, 2021 and including youngsters 12–15 years old. During the time period of Group 1, the dominant wild-type, non-N501Y strain in Israel has been replaced with the B.1.1.7 variant (alpha strain).17 During the time period of Group 2, the dominant strain in Israel was the B 1.617. 2 (delta), Figure S1.
Anonymized data, without any personal identifiers, were used in this analysis in all databases investigated. This study was approved by the Hadassah Hebrew University Hospital institutional review board (IRB approval number HMO-0372-20). The study was exempt from the requirement for informed consent.
2.3 Outcomes
The primary outcome was COVID-19 related death (reported to the Israeli MOH) within 60 days after the first positive PCR date. Secondary outcomes were: (1) Hospitalization for COVID-19 (hospitalization in COVID-19 designated department, at the time interval between 2 days before PCR-positive and 21 days thereafter, Figure S2; (2) Hospitalization duration. To measure the effect on the hospitalization related (secondary) outcomes we performed an additional independent matching between recently injected and unvaccinated groups. This matching was done without taking into account the first status at hospital admission variable.
2.4 Statistical analysis
For estimating the treatment effect in this study and to produce inferences that are more robust and less sensitive to modeling assumptions, we perform propensity score matching15, 18 so that we can further control for measured confounding variables, namely the gender, age, first status at hospital admission and the date of the first positive PCR. We followed the propensity score matching (15) when a binary treatment is in the study (recently injected [vaccinated] vs. unvaccinated). In detail, we adopted a nearest neighbor (NN) matching on the propensity score (in the study it was estimated using logistic regression), which is commonly assumed appropriate for estimating the average treatment effect in the treated (ATT) when a simple treatment is considered. The method is commonly called 1:1 NN as one-by-one, each treated unit is paired with an available control unit that has the closest propensity score to it. Any remaining control units are left unmatched and excluded from further analysis. Due to the theoretical balancing properties of the propensity score described by Rosenbaum PR, and Rubin DB,19 propensity score matching is an effective way to achieve covariate balance in the treatment groups. The goal of matching is to produce covariate balance, that is, for the distributions of covariates in the two groups to be approximately equal to each other, as they would be in a successful randomized experiment. The importance of covariate balance is that it allows for increased robustness to the choice of model used to estimate the treatment effect; in perfectly balanced samples, a simple difference in means can be a valid treatment effect estimate (loveplot: https://ngreifer.github.io/cobalt/reference/love.plot.html). We are aware of the criticisms to propensity scores.20 They are mostly related to the type of treatment that is in the study. When treatments are multilevel or time evolving simple propensity score matching is not effective. While here, given the binary nature of the treatment, it is effective. Survival curves for the recently injected and unvaccinated groups were estimated using the Kaplan–Meier estimator21, 22 analyzing death, and hospitalization probability. Fisher exact and McNemar tests were used to assess the association between vaccination status and the main outcomes distinguishing by age group, gender and first status at hospital admission. We calculated odds ratio (OR) and 95% confidence interval (CI). As a further remark, though the vaccinated/unvaccinated variable defines unpaired groups, the matched samples mimic the paired ones and, accordingly, the McNemar's test can be used as an alternative to Fisher test, even if the latter is considered more conservative (one example is in Fagerland et al.23 The Fisher exact test results are reported in the Supporting Information Material (in the text, upon depicting p values it refers to McNemar).
A negative binomial generalized linear model was estimated to evaluate the relation between duration of hospitalization and all interactions among age groups, vaccination status and gender. Count data may be distributed as Negative Binomial if the rate, at which events occur, is heterogeneous, and consequently the counts are characterized by overdispersion compared to the Poisson (as typically happens in our length of hospitalization data). The Poisson distribution is nested within the Negative Binomial, in the sense that if no overdispersion/heterogeneity is present, the Negative Binomial distribution converges to the Poisson distribution.
3 RESULTS
3.1 Study population
Our study population included 274 096 Israeli residents that were identified PCR-positive for SARS-CoV-2 during the period between December 20th, 2020 and October 7th, 2021, (Figure 1). Among them, 11,687 were found positive on the same day they received their first vaccine injection (BNT162b2; Pfizer) or on 1 of the 5 days thereafter i.e., “recently injected” group. We matched the recently injected group to unvaccinated controls according to the following variables: age, age group, gender, date and first status at hospital admission (Figure 1). The characteristics of the matched unvaccinated control and the recently injected group are shown in Table 1. Notably, there are no differences in demographic characteristics between the two groups (Figure S2).
| Characteristics | Unvaccinated controls | Recently injected |
|---|---|---|
| Median age (IQR)—year | 38 (24–53) | 38 (24–53) |
| Age group—no. (%) | ||
| 12–34 years | 5252 (44.94) | 5252 (44.94) |
| 35–44 years | 1872 (16.02) | 1872 (16.02) |
| 45–54 years | 1831 (15.67) | 1831 (15.67) |
| 55–64 years | 1320 (11.29) | 1320 (11.29) |
| 65–74 years | 766 (6.55) | 766 (6.55) |
| 75–84 years | 380 (3.25) | 380 (3.25) |
| >85 years | 266 (2.28) | 266 (2.28) |
| Sex—no. (%) | ||
| female | 5678 (48.58) | 5678 (48.58) |
| male | 6009 (51.42) | 6009 (51.42) |
- Abbreviation: IQR, interquartile range.
3.2 Primary outcome
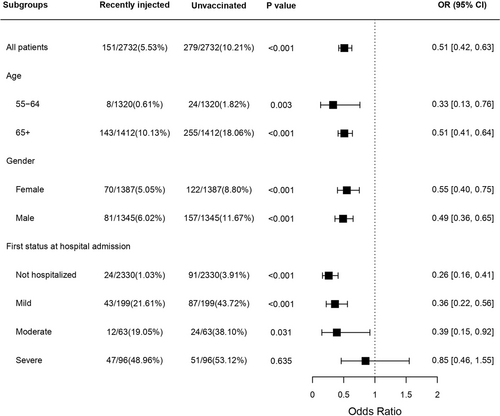
Mortality up to 60 days from first positive PCR, among infected people, 55 years of age and beyond, was significantly lower in the recently injected group compared to the unvaccinated control group (Table 2). In the combined group of patients over 65 years and beyond, male and female, 143 deaths occurred among 1412 recently injected (10.13%) compared to 255 deaths among the 1412 unvaccinated (18.06%), OR 0.51 (95% CI: 0.41–0.64; p < 0.001) (Figure 2). A significant reduction in the death toll was observed among the combined 55–64 age group, with 8 deaths occurring among the 1320 recently injected (0.61%) compared to 24 deaths among the 1320 unvaccinated control (1.82%), OR 0.33 (95% CI: 0.13–0.76; p = 0.007). It should be noted that in the female's sub-group the difference between the recently injected and the unvaccinated groups was not statistically significant, possibly due to a small number of deaths. Under the age of 55, we did not observe a significant difference between the groups (Table S1, Figures S4–S8).
| Age group | Gender | Recently injected | Unvaccinated | Odd ratio (95% CI) | McNemar p value |
|---|---|---|---|---|---|
| No./total No. of patients (%) | |||||
| 55–64 | female | <5/642 (<0.78) | 7/642 (1.09) | 0.14 (0.00–1.11) | 0.077 |
| male | 7/678 (1.03) | 17/678 (2.51) | 0.41 (0.14–1.04) | 0.034 | |
| 65–74 | female | 8/362 (2.21) | 17/362 (4.70) | 0.46 (0.17–1.14) | 0.066 |
| male | 19/404 (4.70) | 42/404 (10.40) | 0.43 (0.23–0.76) | <0.001 | |
| 75–84 | female | 24/215 (11.16) | 37/215 (17.21) | 0.61 (0.33–1.09) | 0.055 |
| male | 28/165 (16.97) | 54/165 (32.73) | 0.42 (0.24–0.73) | <0.001 | |
| 85+ | female | 37/168 (22.02) | 61/168 (36.31) | 0.50 (0.30–0.82) | 0.002 |
| male | 27/98 (27.55) | 44/98 (44.90) | 0.47 (0.25–0.88) | 0.004 | |
- Abbreviations: CI, confidence interval; MoH, ministry of health.
- a Death numbers under five were written as <5 due to MoH privacy policy.
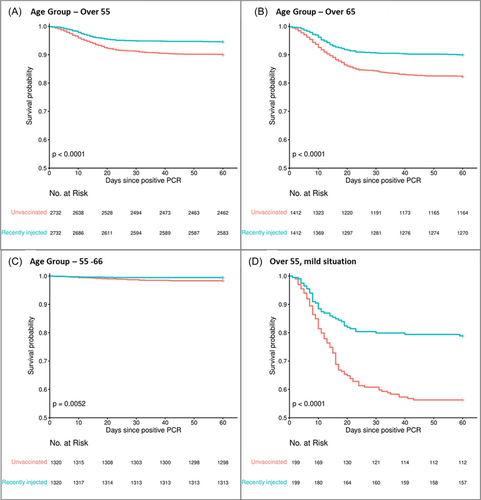
Importantly, we found that death rate was significantly reduced, in the combined group of patients over 55 years and beyond, also among the sub-group of people who were found positive on the same day or 1 day after they received their first vaccine. More specifically, 34 deaths occurred among the 357 recently injected (9.52%) compared to 51 deaths among the 357 unvaccinated control (14.29%), OR 0.63 (95% [CI]: 0.39–1.03; p = 0.022) (Table S2).
To assess the effect of the first status at hospital admission, we divided all participants in the study who were over 55 to sub-groups according to this variable. We found that among the group of patients that arrived at the hospital in mild or moderate status and among the group of patients who were not hospitalized, mortality rate was significantly lower in the recently injected group compared to the unvaccinated control group, with Odd Ratios between 0.26 and 0.39 (Table 3, Figure S9). In the group of patients that arrived at the hospital in severe status, deaths that occurred among the recently injected was also lower than deaths among the unvaccinated but the difference was not statistically significant (OR: 0.85 [0.46–1.55]). The overall survival effect is depicted in the Forest plot as illustrated in Figure 3.
| First status at hospital admission | Recently injected | Unvaccinated | Odd ratio (95% CI) | McNemar p value |
|---|---|---|---|---|
| No./total No. of patients (%) | ||||
| Not hospitalized | 24/2330 (1.03) | 91/2330 (3.91) | 0.26 (0.16–0.41) | <0.001 |
| Mild | 43/199 (21.61) | 87/199 (43.72) | 0.36 (0.22–0.56) | <0.001 |
| Moderate | 12/63 (19.05) | 24/63 (38.10) | 0.39 (0.15–0.92) | 0.031 |
| Severe | 47/96 (48.96) | 51/96 (53.12) | 0.85 (0.46–1.55) | 0.635 |
| Critical | 6/8 (75.00) | 8/8 (100.00) | 0.00 (0.00–5.21) | 0.48 |
| Unknown | <5/21 (<23.81) | <5/21 (<23.81) | 1.00 (0.16–6.33) | 1 |
- Abbreviations: CI, confidence interval; MoH, ministry of health.
- a Death numbers under five were written as <5 due to MoH privacy policy.
3.3 Secondary outcomes
Hospital admissions in the combined group of patients over 65 years and beyond, male and female, was significantly less in the recently injected group compared to unvaccinated group, 321/1412 (22.73%) versus 407/1411 (28.84%), respectively (OR: 0.73, 95% CI: 0.61–0.86; p < 0.001). A similar reduced hospitalization rate was seen in the 55–64 combined group, 81/1320 (6.14%) versus 116/1320 (8.79%), respectively (OR: 0.68 95% CI: 0.50–0.92; p = 0.011) (Table S3, Figures S10–S19). Regarding the analysis on the length of hospitalization, we did not find any statistically significant effect of vaccination status. From our analysis, only age and gender play a role in the estimation of the hospitalization duration, with males and older age groups confirmed to increase the hospitalization length. No other interactions were significant.
In an effort to ratify that the recently injected group compared to unvaccinated group are highly similar in their clinical status at acute COVID-19 infection and demographics, we have undertaken a major investigative effort to confirm that these were highly similar (Suppoting Information Analyses S1 Figures S19–S21).
To eliminate the possibility that the survival difference between the recently injected group and the unvaccinated group was due to differences in their pre-existing health status, we obtained data regarding pre-existing health condition of Covid-19-infected patients and compared between vaccinated (not specifically “recently injected”) and unvaccinated patients using the Charelson index. We found that there was no statistically significant difference between the groups (Suppoting Information Analyses S2).
4 DISCUSSION
The death toll from COVID-19 is rising, and numerous strategies are essential to reduce this mortality rate world-wide. In this report we show that the BNT162b2 vaccine could also be applied to patients as a postexposure vaccine prophylaxis manner, significantly reducing the death burden at the high-risk groups of aged patients by 50%. We propose that this approach could be used with a significant positive impact on survival following SARS-CoV-2 exposure as well as upon hospitalization. There are numerous potential benefits in using this postexposure vaccine prophylaxis approach. In many countries, once an individual is identified as positive by PCR for SARS-CoV-2, an epidemiological investigation is performed to identify those exposed to this COIVID-19 patient. These identified individuals are then isolated without any preventive treatment. We propose that as in other postexposure vaccine prophylaxis infectious events, the exposed individuals should be vaccinated to prevent severe disease culminating in hospitalization and in some cases leading to death. In many countries, the availability of vaccines is low and also in some places there are anti-vaccine environments. One question remains as to whom should the vaccine be provided. It is accepted in many societies that prioritization is to provide the vaccine to the aged, immunosuppressed patients and the medical staff. It could also be beneficial to include in this selected group of individuals, vaccination to SARS-CoV-2-exposed patients. This postexposure vaccine prophylaxis could have an advantage of halting, or at least attenuating, the spread of the virus, possibly by reducing its titer in the respiratory tract of exposed individuals. This approach is substantiated by the fact that infected patients are spreading the virus before the occurrence of symptoms.15, 24 In our investigation we have retrospectively analyzed the effects of comorbidities as depicted in the Suppoting Information Analyses S1 section titled “analysis of comorbidities and status at hospital admission.” We did not identify that these comorbidities were prevalent differently between groups. However, due to the fact that this was a retrospective investigation future studies in a prospective manner should be designed in a way to equilibrate between comorbidities.
Our findings and conclusions are based on multiple statistical methods which make these results robust due to the different approaches to data analysis. Of course, a crucial role is played by the p value, whose correct use has been widely discussed in the literature (Wasserstein & Lazar25 However, as discussed by Wellek,26 Gasparini,27 Piegorsch,28 and Brannath,29) the p value is the decisive criterion in particular when binary decision-making is indispensable, as in our analysis.
Based on our findings, we propose that additional approaches should be investigated to potentially expand the usage of postexposure vaccine prophylaxis to reduce COVID-19 morbidity. Patients who develop severe respiratory symptoms, including low saturated oxygen (<93%), increased respiratory rate (>18/min) and pulmonary infiltrates on chest X-ray, could potentially benefit from a vaccination which would overcome the SARS-CoV-2 infection-related deterioration to pneumonitis and adult respiratory distress syndrome. Recently, an investigation that compared adult and children immune response to SARS-CoV-2 had shown that activation of the immune response in children results in an attenuation of infection.15, 26, 30 Stimulating the interferon response by vaccination could improve the immune reaction, simulating the children natural history of SARS-CoV-2 infection. However, until postexposure vaccine prophylaxis is investigated prospectively in a clinical study, the safety of this approach is unknown. It is well accepted that one important determinant in the outcome of COVID-19 infection is the genetic alterations in interferon pathway genes.15, 30, 31 Postexposure vaccine prophylaxis could skew the immune response to enhance the interferon signaling pathways.15, 30, 32
AUTHOR CONTRIBUTIONS
Study conception and design: Eithan Galun, Giovanna Jona-Lasinio, Antonello Maruotti, Hilla Giladi, Yehuda Warszawer, Omri Or, Sagit Arbel-Alon and Zohar Shmuelian. Data collection: Meytal Avgil Tsadok, Roy Cohen, Galit Shefer, Dekel Shlomi and Moshe Hoshen. Analysis and interpretation of results: Yehuda Warszawer, Zohar Shmuelian, Giovanna Jona-Lasinio, Antonello Maruotti, Sagit Arbel-Alon and Eithan Galun. Draft manuscript preparation: Eithan Galun, Zohar Shmuelian, Hilla Giladi, Giovanna Jona-Lasinio, and Antonello Maruotti. All authors reviewed the results and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Mrs. Nofar Avni, BI Developer from the IT Unit and Mrs. Olga Litinetsky (Slutsky) Head of Data Research Unit, Research Fund of the Hadassah Medical Organization for assistance in data accessibility and retrieval.
The research of Antonello Maruotti and Giovanna Jona-Lasinio has been partially supported by the Ministero dell'Istruzione, dell'Università e della Ricerca, project number FISR2020IP_00156 “Modelli statistici inferenziali per governare l'epidemia” (SMIGE).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
CODE AVAILABILITY
https://github.com/yw7/bnt162b2-post-exposure-prophylaxisagainst-covid-19/blob/main/code.R
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Israel ministry of health. Restrictions apply to the availability of these data, which were used under license for this study. Data are available once an IRB approval is provided to retrieve it. All the data included in this investigation is anonymized and as such will be provided.




