Does ACE2 mediate the detrimental effect of exposures related to COVID-19 risk: A Mendelian randomization investigation
Abstract
Objectives
Adiposity, smoking, and lower socioeconomic position (SEP) increase COVID-19 risk while the association of vitamin D, blood pressure, and glycemic traits in COVID-19 risk were less clear. Whether angiotensin-converting enzyme 2 (ACE2), the key receptor for SARS-CoV-2, mediates these associations has not been investigated. We conducted a Mendelian randomization study to assess the role of these exposures in COVID-19 and mediation by ACE2.
Methods
We extracted genetic variants strongly related to various exposures (vitamin D, blood pressure, glycemic traits, smoking, adiposity, and educational attainment [SEP proxy]), and ACE2 cis-variants from genome-wide association studies (GWAS, n ranged from 28 204 to 3 037 499) and applied them to GWAS summary statistics of ACE2 (n = 28 204) and COVID-19 (severe, hospitalized, and susceptibility, n ≤ 2 942 817). We used inverse variance weighted as the main analyses, with MR-Egger and weighted median as sensitivity analyses. Mediation analyses were performed based on product of coefficient method.
Results
Higher adiposity, lifetime smoking index, and lower educational attainment were consistently associated with higher risk of COVID-19 phenotypes while there was no strong evidence for an association of other exposures in COVID-19 risk. ACE2 partially mediates the detrimental effects of body mass index (ranged from 4.3% to 8.2%), waist-to-hip ratio (ranged from 11.2% to 16.8%), and lower educational attainment (ranged from 4.0% to 7.5%) in COVID-19 phenotypes while ACE2 did not mediate the detrimental effect of smoking.
Conclusions
We provided genetic evidence that reducing ACE2 could partly lower COVID-19 risk amongst people who were overweight/obese or of lower SEP.
1 BACKGROUND
A previous systematic review of Mendelian randomization (MR) studies suggested smoking, obesity, and lower educational attainment (as a proxy of socioeconomic position [SEP]) increase COVID-19 risk, while glycemic traits, blood pressure, and vitamin D are likely by-standers.1 However, the underpinning mechanisms linking these traits with COVID-19 risk have not been thoroughly explored, which has important implications for reducing associated risk in specific high-risk populations.
Given angiotensin-converting enzyme 2 (ACE2) is the protein receptor for SARS-CoV-2 which increases the risk of COVID-19 and its severity,2 it may mediate the detrimental impact of certain exposures on COVID-19 risk. A recent study has also shown that increased plasma ACE2 was associated with higher risk of COVID-19.2 To assess whether ACE2 mediated the detrimental effect of exposures related to COVID-19 risk, we used (MR), a design which is more resistant to confounding than conventional observational studies.3 We first reassessed whether various exposures included in the previous review (e.g., blood pressure, adiposity, vitamin D, glycemic traits, smoking, and educational attainment) impacted COVID-19 risk using the most updated COVID-19 genome-wide association studies (GWAS) summary statistics (Release 7) to rule out possibility of false negative due to insufficient sample sizes in previous MR studies.1 We then examined the impact of these exposures on plasma ACE2, and subsequently quantified the proportion of COVID-19 risk mediated by plasma ACE2.
2 METHODS
This study had three instrumental variable assumptions.3 The instruments should be strongly associated with exposure of interest (Relevance); the instruments should be independent of confounders of exposure-outcome relation (Independence); and the instruments should not be associated with the outcomes independent of its association with the exposure (Exclusion restriction). Figure 1 shows the overall schematic diagram of this study (2-step MR design).
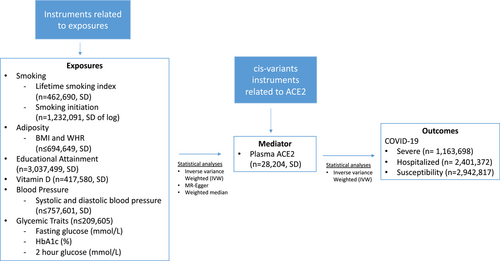
2.1 Exposure
We obtained strong, independent single nucleotide polymorphisms (SNPs) (p < 5 × 10−8; r2 < 0.001) for exposures. These included smoking (lifetime smoking index [n = 462 690] standard deviation [SD]) and smoking initiation [n = 1 232 091], SD of log)4, 5; adiposity (body mass index [BMI, SD] and waist-to-hip ratio [WHR, adjusted for BMI, SD] [n ≤ 694 649]),6, 7 and educational attainment (n = 3 037 499, SD).8 We also included systolic and diastolic blood pressure (n ≤ 757 601, SD [converted from reported mmHg based on pooled SD in studies of the blood pressure GWAS]),9 glycemic traits (fasting glucose [mmol/L], glycated hemoglobin [HbA1c, %], and 2-h glucose [mmol/L] [n ≤ 209 605]),10 and vitamin D (n = 417 580, SD)11 although smaller MRs suggested these traits were unrelated to COVID-19 risk.1
2.2 Mediator
The mediator was plasma ACE2 (SD), measured by proximity extension assay technology (n = 28 204).2 When assessing the association of mediator in COVID-19, we only selected cis-variants near ACE2 (rs1849863, rs143380244, and rs73202884, as identified in the original GWAS)2 which were less vulnerable to horizontal pleiotropy compared to trans-variants. These variants were not in high linkage disequilibrium (r2 < 0.0027) based on LD link (https://ldlink.nci.nih.gov/?tab=home).
2.3 Outcome
The outcomes were COVID-19 phenotypes (susceptibility, hospitalised, and very severe COVID-19), obtained from the summary statistics of COVID-19 Host Genetics Initiative [Release 7, April 2022, n ≤ 2 942 817], https://www.covid19hg.org).12 COVID-19 status were defined differently across the studies. In brief, susceptibility to COVID-19 was defined as having laboratory-confirmed SARS-CoV-2 infection, self-reports, or diagnosis made by physicians/hospitals. Hospitalized COVID-19 was defined as hospitalized laboratory-confirmed SARS-CoV-2 infection, with related corona-related symptoms as the reason for hospitalization. Severe COVID-19 was defined as the condition of hospitalized COVID-19 and death/respiratory support.
All of the above GWAS were predominantly derived from participants of European descent.
2.4 Statistical analyses
Data harmonization was performed to ensure the effect alleles were aligned properly across the summary statistics data from different GWAS, based on effect alleles and effect allele frequencies (for palindromic SNPs). Palindromic SNPs with minor allele frequency above 0.42 were removed to avoid ambiguity in strand direction.
We assessed the association of exposures in COVID-19 risk (Exposure to Outcome) using inverse variance weighted (IVW) with multiplicative random effect. We computed I2 for Wald ratio heterogeneity, MR-Egger intercept for overall horizontal pleiotropy, and F statistics for instrument strength. We included sensitivity analyses which relied on different assumptions to strengthen causal inference. These included weighted median method which gave valid estimates when more than 50% of the weights are derived from valid instruments; and MR-Egger which gave valid estimates even if all instruments were invalid, as long as the instrument strength independent of direct effect (InSIDE) assumption holds.13 We also assessed (1) the association of these exposures with plasma ACE2 (Exposure to Mediator) using IVW and sensitivity analyses and (2) the association of plasma ACE2 in COVID-19 risk using IVW given only cis-variants were used (Mediator to Outcome). For exposures associated with plasma ACE2, we calculated the indirect effect using the product of coefficient method, and estimated the proportion mediated.14 The corresponding standard errors were obtained using the delta method.
All analyses were performed using R Version 4.0.4 (R Core Team [2021]).
This study only used publicly available data and hence no ethical approval was required. Details of ethical approval and participant consent for each of the studies that contributed to the GWAS can be found in the original publications.
3 RESULTS
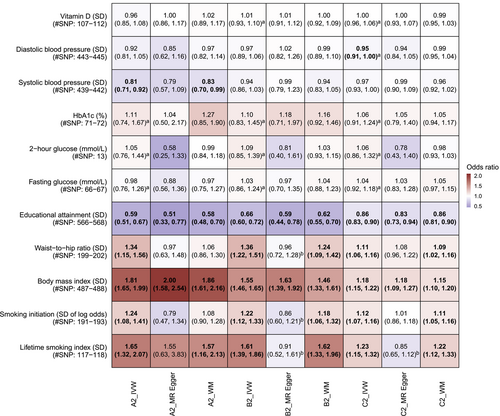
The F statistics of the instruments were all >10, indicating low weak instrument bias (From 22 to 8036). In Figure 2, lifetime smoking index, higher adiposity, lower educational attainment were consistently associated with COVID-19 phenotypes, but not vitamin D and glycemic traits. I2 did not indicate severe heterogeneity but MR-Egger intercept indicated potential biases in some analyses, such as the association of WHR in hospitalized COVID-19 risk where the MR-Egger estimate was close to null. Sensitivity analyses generally produced consistent findings for educational attainment and BMI for all COVID-19 phenotypes, and lifetime smoking index for severe COVID-19 only. There was an inverse association for systolic blood pressure in severe COVID-19 risk, but not other COVID-19 phenotypes.
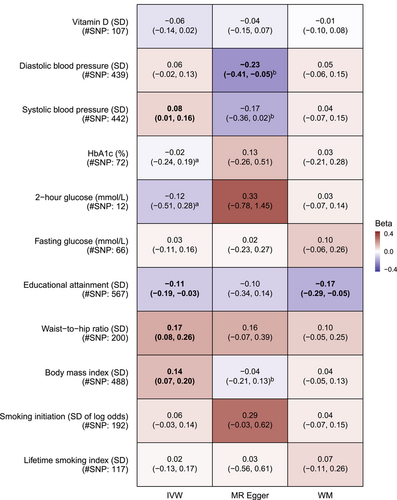
In Figure 3, higher BMI (0.14 per SD, 95% confidence interval [CI]: 0.07–0.20) and WHR (0.17 per SD, 95% CI: 0.08–0.26) were associated with higher plasma ACE2, while higher educational attainment was associated with lower plasma ACE2 (−0.11 per SD, 95% CI −0.19 to −0.03). Sensitivity analyses gave consistent results for educational attainment and WHR. Other phenotypes, including lifetime smoking index which was associated with higher risk of COVID-19, were not related to plasma ACE2.
In Table 1, plasma ACE2 was positively associated with all COVID-19 phenotypes (ORSevere: 1.21 per SD, 95% CI: 1.11–1.32; ORHospitalized: 1.21 per SD, 95% CI: 1.13–1.30; ORSusceptibility: 1.11 per SD, 95% CI: 1.03–1.19). Table 1 also shows the proportion mediated from BMI, WHR, and educational attainment to COVID-19 phenotypes by plasma ACE2. The mediation analysis showed that proportion of association mediated by plasma ACE2 ranged from 4.3% to 8.2% for BMI, 10.7% to 16.8% for WHR, and 4.0% to 7.5% for educational attainment.
| (A): ACE2->COVID-19 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Severe COVID-19 (OR, 95% CI) | Hospitalized COVID-19 (OR, 95% CI) | COVID-19 Susceptibility (OR, 95% CI) | |||||||
| ACE2 (SD) | 1.21 (1.11–1.32) | 1.21 (1.13–1.30) | 1.11 (1.03–1.19) | ||||||
| (B) Proportion of association mediated by ACE2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Severe COVID-19 | Hospitalized COVID-19 | COVID-19 Susceptibility | |||||||
| Total effect (OR, 95% CI) | Indirect effect (OR, 95% CI) | Proportion mediated (%, 95% CI) | Total effect (OR, 95% CI) | Indirect effect (OR, 95% CI) | Proportion mediated (%, 95% CI) | Total effect (OR, 95% CI) | Indirect effect (OR, 95% CI) | Proportion mediated (%, 95% CI) | |
| Body mass index (SD) | 1.81 (1.65, 1.99) | 1.03 (1.00, 1.05) | 4.34 (0.89, 7.78) | 1.55 (1.46, 1.65) | 1.03 (1.00, 1.05) | 5.93 (1.66, 10.20) | 1.18 (1.15, 1.22) | 1.01 (1.00, 1.03) | 8.24 (0.03, 16.45) |
| Waist-to-hip ratio (SD) | 1.34 (1.15, 1.56) | 1.03 (1.00, 1.07) | 11.15 (5.93, 16.36) | 1.36 (1.22, 1.51) | 1.03 (1.00, 1.06) | 10.69 (4.67, 16.71) | 1.11 (1.06, 1.16) | 1.02 (1.00, 1.04) | 16.75 (3.64, 29.85) |
| Educational attainment (SD) | 0.59 (0.51, 0.67) | 0.979 (0.974, 0.984) | 4.00 (3.92, 4.09) | 0.66 (0.60, 0.72) | 0.98 (0.97, 0.99) | 5.16 (4.64, 5.67) | 0.86 (0.83, 0.90) | 0.9887 (0.9886, 0.9888) | 7.53 (5.54, 9.52) |
- Abbreviations: ACE2, angiotensin-converting enzyme 2; CI, confidence interval; OR, odds ratio; SD, standard deviation.
4 DISCUSSION
Similar to previous MR studies,1 our study suggested that smoking, higher adiposity, and lower educational attainment were associated with higher COVID-19 risk and its severity. As expected, phenotypes unrelated to COVID-19 were unrelated to plasma ACE2. Plasma ACE2 partially mediated the effects of adiposity and educational attainment but not the effect of smoking.
Previous studies showed obesity increases COVID-19 risk.1, 15 However, the mechanism remains unclear where adjustment for proinflammatory responses, a postulated mediator, did not attenuate the association.15 Our study provides genetic evidence that plasma ACE2 mediates part of its detrimental effects although such pathway is more relevant to central adiposity (WHR) than general adiposity (BMI). One implication is that if weight loss programmes are not effective due to noncompliance, plasma ACE2 could be an alternative therapeutic target to mitigate harms associated with adiposity. The same pathways may also explain the detrimental effect of lower education in COVID-19 risk given its co-occurrence with obesity.
Our analyses also indicated possible harm of smoking in all COVID-19 phenotypes, consistent with our previous MR study.16 In terms of mechanisms, our study differed from a previous MR study showing a possible relation of smoking in ACE2 expression in tissues.17 However, gene expression does not always strongly correlate with protein abundance, which may have explained the discrepancies between our study and the previous study.18 A previous MR study had also explored other possible mechanisms for COVID-19 risk, such as lung function and liability to chronic obstructive pulmonary disease, but showed no such evidence.16 Whether other unexplored pathways, such as behavioural aspects of smoking (e.g., mask-off), contribute to increased susceptibility to COVID-19 warrant further investigations. It is noted that there were few observational studies suggesting an inverse association of smoking in COVID-19 severity.19 However, these studies are potentially invalid due to selection bias and sparse data bias in covariate adjustments.20
Strengths of this study included the use of the largest available GWAS for plasma ACE2 and COVID-19, and MR to reduce confounding. However, limitations included possible violation of the MR assumptions (e.g., selection bias for exposures [e.g., blood pressure] related to survivorship),21 the presence of potential outliers leading to directionally different associations for MR-Egger), smaller sample size for plasma ACE2, and lack of multivariable MR in mediation analyses due to limited variants related to plasma ACE2. Although potential misclassification of COVID-19 status due to the use of self-reports as case ascertainment method for some studies in the GWAS could attenuate our estimates, the expected association of ACE2 in COVID-19 risk in this study suggested misclassification bias is likely not severe.
In conclusion, we showed plasma ACE2 mediates the detrimental effect of adiposity and lower educational attainment in COVID-19 risk, and shed light on corresponding mechanistic pathways and possible target of intervention for relevant subpopulations.
AUTHOR CONTRIBUTIONS
Shiu Lun Au Yeung and Kin On Kwok conceptualized the idea. Tommy Hon Ting Wong conducted the analyses, with supervision by Shiu Lun Au Yeung Shiu Lun Au Yeung wrote the first draft of the manuscript with critical feedback and revisions from Tommy Hon Ting Wong, Baoting He, Shan Luo, and Kin On Kwok. All authors gave final approval of the version to be published. Shiu Lun Au Yeung had primary responsibility for final content.
ACKNOWLEDGMENTS
We were grateful to the ACE2 GWAS investigators, MAGIC consortium, and COVID-19 Host Genetics Initiatives (https://www.covid19hg.org/) for providing the summary statistics used in this study. We thank Ms. Li Ho Yi Queenie for constructing Figure 1. There is no funding related to this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data used in this study are publicly available and can be accessed via the information stated in the cited references and websites.




