COVID-19 vaccinations and rates of infections, hospitalizations, ICU admissions, and deaths in Europe during SARS-CoV-2 Omicron wave in the first quarter of 2022
Abstract
The vaccination campaigns brought hope to minimizing the coronavirus disease 2019 (COVID-19) burden. However, the emergence of novel, highly transmissible Omicron lineage of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the waning of neutralizing antibodies a few months after vaccination has brought concerns over the vaccine efficacy. The present work analyzed the relationships between COVID-19 vaccine coverage (completion of primary course and booster dose intake) in the European Economic Area and rates of infection, hospitalizations, admissions to intensive care units (ICU), and deaths during the Omicron wave in the first quarter of 2022 (January–April). As demonstrated, infection rates were not correlated to vaccine coverage in any considered month. For January and February, the rates of hospitalizations, intensive care unit (ICU) admissions, and death due to COVID-19 were strongly negatively correlated (r =− 0.54 to −0.82) with the percentage of individuals who completed initial vaccination protocol and the percentage of those who received a booster dose. However, in March and April, the percentage of the population with primary vaccination course correlated negatively only with ICU admissions (r = −0.77 and −0.46, respectively). The uptake of boosters in March still remained in significant negative correlation with hospitalizations (r = −0.45), ICU admissions (r = −0.70) and deaths due to COVID-19 (r = −0.37), although in April these relationships were no longer observed. The percentage of individuals with confirmed SARS-CoV-2 infection did not correlate with the pandemic indices for any considered month. The study indicates that COVID-19 vaccination, including booster administration, was beneficial in decreasing the overwhelming of healthcare systems during the Omicron wave, but novel vaccine strategies may be required in the long term to enhance the effectiveness and durability of vaccine-induced protection during future waves of SARS-CoV-2 infections.
1 INTRODUCTION
The coronavirus disease 2019 (COVID-19) vaccination campaigns in Europe were launched at the beginning of 2021 and were met with varying levels of vaccine hesitancy.1-3 Nevertheless, by the end of the year, 69% of the population in the European Economy Area (EEA) completed the initial vaccination protocol consisting of two doses of BNT162b2 (BioNTech/Pfizer, mRNA-1273 (Moderna) or AZD1222 (Oxford/AstraZeneca), or a single dose of AD26.COV2.S (Janssen/Johnson & Johnson).4 Although the use of particular vaccines in the EEA varied, while the vaccination protocols (e.g., regarding a gap between doses) were subject to temporal changes depending on vaccine availability in selected countries, the BNT162b2 was the most often administrated product in the EEA countries.4
Due to the emergence of novel, more transmissible, and severe variants such as B.1.617.2 (Delta),5, 6 and observation of gradual decline of serum antibody levels a few months after the last vaccine dose,7, 8 the booster strategies, based mostly on messenger RNA (mRNA) vaccines (BNT162b2 and to a lesser extent – mRNA-1273), were adopted by various EEA countries since September 2021. According to the European Centre for Disease Prevention and Control (ECDC), 35% of the EEA population have already received a booster dose of the COVID-19 vaccine by the end of 2021.4 However, booster programs were met with varying hesitancy in European countries, particularly among younger individuals, with the main reasons against accepting booster COVID-19 vaccine doses including side effects experienced after previous doses, safety uncertainties, and the opinion that further vaccination is unnecessary.9-11 As a result, varying uptake of booster dose was seen in the EEA, with the highest rates, exceeding 60% in April 2022, observed in Belgium, Germany, Italy, and Portugal, while the lowest (<10%) in Bulgaria and Romania (Figure 1).
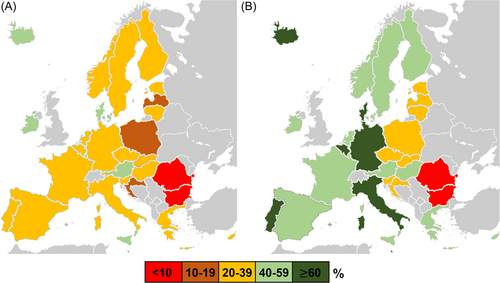
The will for vaccination, be it primary or with a booster dose, was likely also altered by the emergence of the B.1.1.529 (Omicron) lineage, first identified in November 2021 in Botswana and South Africa.12 With over 50 sense mutations accumulated in the genome, approximately 30 concerning spike protein and 10 in the receptor-binding domain, it soon became clear that Omicron is efficient in evading vaccine-induced humoral immunity, a primary cause of its rapid worldwide spread.13-16
In some European countries, Omicron was already the most prevalent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lineage in December 2021, while in January 2022 it became dominant in EEA.17 At the same time, the infections with the Omicron variant were characterized by substantially lower clinical severity and statistically decreased odds for hospitalization, oxygen supplementation, and death.18, 19 These observations have led to assumptions that high vaccine coverage, including uptake of booster doses, is no longer necessary. On the other hand, despite lesser clinical severity, Omicron could still induce high rates of hospitalizations and mortality due to its significantly increased transmissibility compared to previously dominating SARS-CoV-2 variants. The odds of hospitalization due to Omicron infection were higher in unvaccinated adults, adolescents, and children.18, 20, 21 Moreover, unvaccinated patients hospitalized with COVID-19 caused by Omicron were characterized by greater disease severity.22 Therefore, vaccines remained to offer a significant level of protection from severe COVID-19.18, 23, 24 However, a booster dose's importance in improving humoral and cellular immunity was postulated to be greater than in the case of Delta variant.22, 25
The present study analyzed whether vaccine coverage in the EEA countries, including completion of initial vaccination protocol and uptake of a booster dose, was associated with COVID-19 burden (i.e., rates of infections, hospitalizations, admission to intensive care units [ICU], and deaths) during the wave of Omicron variant in the first quarter of 2022.
2 MATERIALS AND METHODS
The data on confirmed SARS-CoV-2 infections, hospitalizations, ICU admission, and death due to COVID-19 during the SARS-CoV-2 wave in the first quarter of 2022 (January–April) was retrieved for each EEA country from ECDC. According to the ECDC which is sourcing its data from TESSy and the GISAID EpiCoV databases, in January 2022 the Omicron variant was identified in all EEA countries with an estimated prevalence of 69.4%.17, 26 The following parameters were then calculated:
In the case of rates of hospitalizations (Formula 3) and ICU admissions (Formula 4), the number of patients reported daily was not summarized to avoid counting the same patients multiple times as ECDC data shows the total number of patients in hospitals/ICU for each day of the month. Therefore, for these two parameters, the calculation of daily means for each month was only feasible. The size of the population of each country was sourced from Eurostat.27
Data on the percentage of individuals in each country who completed the initial COVID-19 vaccination protocol and individuals who received a booster dose were retrieved from the ECDC Vaccine Tracker4 for the first day of each considered month was used (or, if not provided, the nearest day for which the data was available). Similarly, the percentage of individuals in the population with identified SARS-CoV-2 infection was collected. The relationship between vaccination coverages, percentage of convalescents, and pandemic indices in the EEA were assessed for each month with the Pearson correlation coefficient (r). A p-value < 0.05 was considered statistically significant. The following classification of correlation strengths was applied (relevant only if p < 0.05): r = 0.1 to 0.3 or −0.1 to −0.3 – very weak; r = 0.3 to 0.5 or −0.3 to −0.5 – weak; r = 0.5 to 0.7 or −0.5 to −0.7 – moderate; r = 0.7 to 1.0 or r = −0.7 to −1.0 – strong.28
3 RESULTS
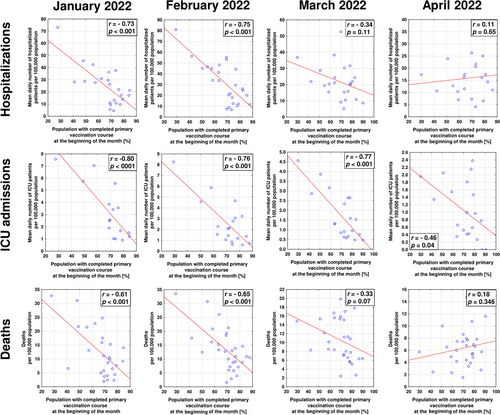
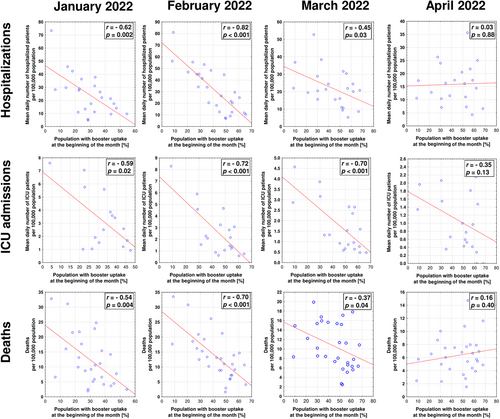
No significant relationships between identified SARS-CoV-2 infections and vaccine coverage (including the percentage of populations with completed initial protocol and percentage with booster uptake) were found for any of the considered months (p > 0.05 in all cases; Table S1). However, for January and February, rates of hospitalizations, ICU admissions, and deaths due to COVID-19 were significantly negatively correlated with both indices of vaccine coverage (Figures 2 and 3). In March and April, the percentage of the population with completed initial protocol correlated negatively only with ICU admissions (Figure 2). The uptake of boosters in March negatively correlated with hospitalizations, ICU admissions, and deaths due to COVID-19, while in April, these relationships were no longer present (Figure 3). The percentage of the population with confirmed SARS-CoV-2 infection did not correlate significantly with rates of infections (except for February, r = 0.40, p = 0.027 and March, r = 0.46, p = 0.011), hospitalizations, ICU admissions, and deaths due to COVID-19 (Table S1).
4 DISCUSSION
The present study provides additional epidemiological data on the effectiveness of COVID-19 vaccinations, including the administration of booster dose in EEA. Its understanding is important due to the rapid emergence and spread of Omicron lineage, characterized by the substantially increased immune escape compared to other SARS-CoV-2 variants.
For any considered month, vaccination coverage, including completion of initial protocol and uptake of a booster dose, was not associated with infection rate in EEA. Our previous study has documented the significant negative correlations between infections and vaccination status in EEA countries during autumn 2021, dominated by Delta variant.29 However, such a protective effect was already lost in December 2021, and we suggested that it was due to the emergence of the Omicron variant.26, 29 The present research confirms it and further indicates that a higher intake of booster dose may not necessarily lead to a lower number of Omicron infections. A number of studies have indicated that compared to preceding SARS-CoV-2 variants, the Omicron subvariants are better adapted to evade vaccine-induced humoral immunity. Even though booster dose administration substantially increases neutralizing antibody titers against BA.1 and BA.2 subvariants, this effect is not as profound as in other viral variants.13, 30 The COVID-19 vaccines authorized by the European Medicine Agency in 2020/2021, be it adenoviral or mRNA, were based on the spike protein of the SARS-CoV-2 version that emerged in Wuhan, China, in late 2019. As a result, they poorly match the Omicron lineage, which accumulated numerous mutations concerning the spike protein, including approximately 10 only in the receptor-binding domain.12
Therefore, it is debatable if administering a second booster dose of the first-generation COVID-19 vaccine may provide a further advantage in terms of significant protection from Omicron infection. In line with this, some initial observations indicate that benefits in this regard for some groups of individuals may only be marginal.31 Thus, in the future, it may be necessary to introduce a multivariant epitope vaccine, adapted also, but not exclusively, to Omicron lineage.3, 32 However, one should bear in mind that a gradual decrease in antibodies observed within months from dose administration will remain a challenge unless the vaccine, more durable, yet broadly neutralizing immunoglobulins, is developed.
Although no relationship between infection and vaccination rates was found in the present study, it should be stressed that vaccine administration may provide other remarkable benefits. First, it has been shown that higher vaccine coverage suppresses the evolution of SARS-CoV-2.33-35 Secondly, the primary goal of vaccination has always been to decrease the clinical severity of infection as it mitigates hospitalization and death rates and decreases the overwhelming of the healthcare system.36 In this regard, the COVID-19 vaccines were performing well in 2021 with an estimated half a million deaths averted in individuals 60 years and older in the WHO European Region.37 Our previous analyses have evidenced that during the autumn 2021 wave of more clinically severe Delta variant of SARS-CoV-2, vaccination rates in particular EEA countries revealed strong negative correlations with hospitalizations, ICU admissions, and deaths due to COVID-19.29 In the present study, similar negative correlations were observed in January and February, indicating the beneficial effect of vaccination, including uptake of primary course and booster coverage, on decreasing the general COVID-19 burden and pressure it has on healthcare systems.
However, in March and April 2022 the percentage of individuals who completed primary vaccination course was negatively associated only with ICU hospitalizations. Importantly though, these correlations were strong with r-values exceeding 0.7. Although booster coverage remained to be negatively associated with all three indices of severe COVID-19 (hospitalizations, ICU admissions, and deaths), this effect was no longer observed in April. Moreover, already in March, the negative association with deaths due to COVID-19 was weak, with r-value below < 0.5. These worrisome findings may indicate the loss of the protection provided by COVID-19 vaccines, including a booster dose. On the other hand, it is consistent with previous observations indicating that although a booster dose temporarily restores antibody levels and strengthens cellular responses,38 the provided protection from different outcomes (including hospital admission) of Omicron infection starts to wane after 3–4 months from administration.39-41 Considering that in EEA, the booster strategies were initiated in autumn 2021, while many countries did not experience a significant rise in their coverage in the first quarter of 2022, their protective effect on the population level could be expected to wane in April of 2022. All in all, this indicates that additional doses of first-generation, variant-adapted, or entirely novel vaccines may be necessary to maintain high levels of protection from severe COVID-19 during the subsequent waves of SARS-CoV-2 infection caused by omicron subvariants or novel viral variants characterized by substantial immune escape.
Importantly, no negative relationships were seen between all pandemic indices (infections, hospitalizations, ICU admissions, and deaths) and the percentage of the population with a confirmed history of SARS-CoV-2 infection. The only exception was February and March 2022, when weak positive correlations was observed. This may be a result of increased surveillance undertaken by various European countries in response to a peak in Omicron infection wave and an increased rate of detected reinfections. This indicates that immunity acquired naturally during waves of previous viral lineages did not provide sufficient protection from Omicron variants. Other studies have already shown that the risk of reinfection with Omicron is substantially increased compared to other SARS-CoV-2 variants.42, 43 Importantly, the present research potentially indicates that protection against severe disease from the previous infection could also be limited. This emphasizes that the Omicron variant should not be regarded as a “natural vaccine” for those who underwent infection before its emergence. The potential importance of vaccination of convalescents in decreasing disease severity should be stressed.44
Study limitations should be emphasized. The correlations demonstrated here do not necessarily imply causation. Although numerous studies evidence the efficacy of COVID-19 vaccines, including a booster dose, in decreasing the severity of disease induced by the Omicron variant, non-pharmaceutical interventions such as face masking, adherence to sanitary measures, and testing capacities may also influence investigated parameters, especially infection rates. Similarly, rates of severe COVID-19 and deaths may not solely be influenced by vaccinations but also by the healthcare system's capacity, which varies across EEA countries,45 and demographical (e.g., age structure of population) and environmental (e.g., pollution levels) factors,46, 47 which were not considered. Furthermore, the study analyzed correlations, which does not necessarily imply causation. Moreover, this study included the percentage of the population confirmed through testing to be infected with SARS-CoV-2, while undetected infections were estimated to comprise more than 50% of the total number of infections.48, 49 Moreover, this study was not designed to understand whether the effect of vaccinations on pandemic indices could be influenced by differences in the applied vaccine doses or vaccination protocols between various EEA countries. One should however note that COVID-19 vaccination campaigns were based mostly on the same vaccine types, with BNT162b2 being the primary one for the initial and booster course.4 Last but not least, the present study did not differentiate between particular Omicron sub-lineages. During the considered period, there was a shift in dominance from BA.1 to BA.2 subvariant, which not only differed in the genetic makeup but was characterized by a higher risk of reinfection and evasion of vaccine-induced humoral immunity, and ultimately greater transmissibility.30, 50 Importantly, the clinical data did not suggest a significant difference in disease severity between these two subvariants; therefore, it is unlikely that changes in the prevalence of BA.1 and BA.2 in EEA during the considered period would influence the hospitalization and mortality rates.51
In summary, the present correlation study indicates that vaccination was related to the decreased COVID-19 burden in the EEA during the first 2 months of 2022 and in March in the case of the booster dose. However, this effect appeared to wane on the population level in April. The study adds to accumulating evidence that additional vaccine doses of next-generation COVID-19 vaccines, possibly adapted to different SARS-CoV-2 variants, may be required to maintain high protection levels during future waves of viral infection.
AUTHOR CONTRIBUTIONS
Conceptualization: Piotr Rzymski. Methodology: Piotr Rzymski and Barbara Poniedziałek. Formal analysis: Nadiia Kasianchuk, Dominika Sikora, Piotr Rzymski, and Barbara Poniedziałek. Writing–original draft: Piotr Rzymski and Barbara Poniedziałek.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the following resources available in the public domain:
- ECDC Vaccine Tracker, https://www.ecdc.europa.eu/en/publications-data/covid-19-vaccine-tracker
- ECDC, https://www.ecdc.europa.eu/en/publications-data/data-daily-new-cases-covid-19-eueea-country
- Eurostat, https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_pjan%26lang=en




