Decreased frequency of a novel T-lymphocyte subset, CD3+CD4−CD7+CD57− T cells, in hepatitis B virus-related end-stage liver disease might contribute to disease progression
Xueping Yu, Yijuan Zheng, and Dawu Zeng contributed equally to this study.
Abstract
CD7 and CD57 are related to the differentiation and functional stages of CD8+ T cells. However, the role of their combined presence in CD8+ T cells in patients with chronic hepatitis B virus (HBV) infection, especially those with end-stage liver disease, remains unclear. Blood samples from healthy volunteers and patients with chronic hepatitis B were analyzed via Luminex assay and ELISA to measure plasma cytokine levels. Further, recombinant IL-22 was used to stimulate peripheral blood mononuclear cells from healthy volunteers, and the frequency of CD3+CD4−CD7+CD57− T cells and apoptosis rates were investigated via flow cytometry. Patients with end-stage liver disease, particularly those with acute to chronic liver failure, showed decreased CD3+CD4−CD7+CD57− T cell frequency. Furthermore, the prevalence of CD3+CD4−CD7+CD57− T cells was negatively correlated with disease severity, prognosis, and complications (ascites). We also observed that IL-22 promoted apoptosis and brought about a decrease in the number of CD3+CD4−CD7+CD57− T cells in a dose-dependent manner. CD3+CD4−CD7+CD57− T cells displayed a B and T lymphocyte attenuator (BTLA)highCD25highCD127high immunosuppressive phenotype and showed low interferon-γ, tumor necrosis factor-α, granzyme A, and perforin expression levels. The present findings will elucidate the pathogenesis of HBV-related end-stage liver disease and aid the identification of novel drug targets.
1 INTRODUCTION
Hepatitis B virus (HBV) infection has become widespread, and in China, approximately 70 million people have HBV infection, with approximately 20 million of these suffering from chronic hepatitis B (CHB), which is more likely to lead to end-stage liver illnesses, such as liver cirrhosis and liver failure.1 Although HBV does not directly destroy liver cells, the immunological response it induces is the primary factor in liver damage and inflammation.2 HBV-specific CD8+ T cells control viral infection and directly kill virus-infected hepatocytes that contribute to liver pathology.3 However, the role of CD8+ T cells and their subsets in CHB pathogenesis has not yet been fully clarified.
Diverse memory populations and developed effector T cells express the terminal differentiation/exhaustion marker, CD57.4 Furthermore, after frequent contact with antigens, activated CD28+CD57−CD8+ T cells may reduce CD28 expression and express CD57. Thus, they acquire the phenotype of terminally differentiated CD28−CD57+CD8+ T cells.5 Further, the frequency of CD8+CD57+ T cells, which express high levels of interferon (IFN)-γ, granzyme B, and perforin6 is increased in various diseases, including AIDS and tuberculosis, suggesting that the protracted accumulation of CD8+CD57+ T cells is a hallmark of several infections and might reflect an end-stage differentiation phenotype.7, 8 Conversely, CD28−CD57−CD8+ T cells, which are more like “early” highly differentiated cells given that they produce tumor necrosis factor (TNF) and IFN-γ to a lesser extent, show a worsened response to DNA damage and are more prone to apoptosis.9
The first distinct T cell lineage marker, CD7, a 40 kDa glycoprotein, is expressed throughout T cell development, and costimulatory signals from CD7 enhance the growth of T cells.10 Several clinical disorders have been associated with the absence of CD7 expression in T cells, indicative of a distinct and persistent differentiation state that develops late during immune response.11, 12 Additionally, naive and memory cells are found in CD7highCD8+ T cells, while effector cells are found in CD7−/lowCD8+ T cells.10
Therefore, it has been proposed that the distinct functional phenotypes of T lymphocytes are represented by the presence or absence of CD7 or CD57. Additionally, a previous study showed that CD7−CD57+ T lymphocytes represent a novel subset of CD4+ T cells that may contribute to immune dysfunction during HIV infection.13 However, the coexpression of CD7 and CD57 in CD8+ T cells has not yet been investigated in HBV infection. Therefore, we investigated CD7 and CD57 expression in CD8+ T cells in patients with CHB, particularly individuals with end-stage liver disease, to determine their functional phenotypes and relationships with disease severity, prognosis, and comorbidities.
2 METHODS
2.1 Patients
Fifty-eight patients with CHB and 40 healthy people (normal controls, NC; without any visible illnesses) participated in this study. All the patients received diagnoses based on a previously described criteria12, 13 and were classified into the following four groups14, 15: (1) asymptomatic carriers (ASC): positive for hepatitis B surface antigen (HBsAg), with normal alanine aminotransferase (ALT) level, and minimal or no evident inflammation observed following liver biopsy; (2) patients with CHB: HBsAg detection for >6 months, hepatitis-related clinical manifestations, histological confirmation of hepatitis, and abnormal ALT levels (≥40 U/L); (3) patients with HBV-related liver cirrhosis (HBV-LC): hepatic dysfunction and portal hypertension, manifestations, such as ascites, jaundice, esophageal varices, and splenomegaly, and diagnosed via histopathology or ultrasonography; and (4) patients with HBV-related acute-on-chronic liver failure (HBV-ACLF): typically characterized by a history of CHB or liver cirrhosis, blood total bilirubin (TBil) levels more than fivefold the normal upper limit, an international normalized ratio (INR) ≥1.5, and a prothrombin time activity <40%. Patients were excluded if they were coinfected with other viruses (e.g., HAV, HCV, HDV, and HIV), had drug- or alcohol-induced liver disease, were treated with antivirals or immunomodulating agents, or had a liver tumor. The clinical characteristics of the patients are listed in Table 1. The study protocol was approved by the ethics committee of Fujian Medical University Affiliated First Quanzhou Hospital.
| Group | NC | ASC | CHB | HBV-LC | HBV-ACLF |
|---|---|---|---|---|---|
| (n = 34) | (n = 11) | (n = 67) | (n = 19) | (n = 32) | |
| Sex (male, %) | 10 (29.41%) | 4 (36.36%) | 52 (77.61%) | 14 (73.68%) | 26 (81.25%) |
| Age (years) | 30.27 ± 1.31 | 30.12 ± 1.40 | 32.79 ± 1.24 | 47.68 ± 3.02 | 47.00 ± 2.10 |
| ALB (g/L) | - | 44.15 ± 0.84 | 40.53 ± 0.49 | 32.73 ± 1.64 | 29.00 ± 0.90 |
| TBIL (umol/L) | - | 14.75 ± 1.71 | 43.82 ± 7.14 | 47.48 ± 8.71 | 296.19 ± 26.32 |
| ALT (IU/L) | - | 23.16 ± 2.36 | 353.56 ± 34.15 | 194.42 ± 56.52 | 488.33 ± 94.34 |
| AST (IU/L) | - | 23.16 ± 1.86 | 202.80 ± 30.21 | 167.11 ± 36.93 | 352.48 ± 63.52 |
| ALP (IU/L) | - | 62.19 ± 13.12 | 100.44 ± 4.82 | 136.83 ± 13.53 | 135.58 ± 9.70 |
| GGT (IU/L) | - | 26.79 ± 14.47 | 130.49 ± 12.61 | 118.33 ± 26.00 | 107.60 ± 14.08 |
| CHE (U/L) | - | 8329.33 ± 419.41 | 6346.66 ± 251.76 | 4056.89 ± 511.06 | 3102.14 ± 237.02 |
| HBsAg (IU/ml) | NA | 20122.13 ± 3452.12 | 18748.81 ± 3270.54 | 3031.98 ± 1082.78 | 10795.32 ± 4280.26 |
| HBeAg n (%) | NA | 4 (36.36%) | 51 (76.12%) | 12 (63.16%) | 17 (53.13%) |
| HBV DNA (Lg copies/ml) | NA | 7.43 ± 1.26 | 7.43 ± 0.19 | 5.69 ± 0.44 | 5.69 ± 0.48 |
| Neut (109/L) | - | 4.75 ± 0.72 | 3.04 ± 0.52 | 2.50 ± 0.39 | 5.42 ± 0.77 |
| Hgb | - | 140.00 ± 5.07 | 142.27 ± 2.38 | 131.17 ± 4.10 | 124.41 ± 3.18 |
| PLT (109/L) | - | 216.89 ± 13.68 | 176.15 ± 5.87 | 86.39 ± 11.22 | 105.87 ± 9.57 |
| PT (s) | - | 11.61 ± 0.37 | 16.68 ± 0.17 | 14.40 ± 0.50 | 23.12 ± 0.09 |
| INR | - | 1.05 ± 0.04 | 1.06 ± 0.02 | 1.30 ± 0.04 | 1.94 ± 0.09 |
| CRP (g/L) | - | - | 4.09 ± 0.51 | 14.80 ± 6.54 | 22.88 ± 6.35 |
| MELD score | - | - | - | 19.00 ± 0.63 | 28.04 ± 0.89 |
| Child-pugh score | - | - | - | 6.37 ± 0.71 | 9.23 ± 0.34 |
| Good prognosis n (%) | - | - | - | 17 (89.47%) | 20 (62.50%) |
| Bacterial infection n (%) | - | - | - | 5 (45.45%) | 13 (40.63%) |
| Ascites n (%) | - | - | - | 5 (45.45%) | 15 (46.88%) |
| Hypersplenism n (%) | - | - | - | 11 (57.89%) | 10 (31.25%) |
- Note: Results are expressed as means ± SEM.
- Abbreviations: ALB, albumin; ALP, Alkaline phosphatase; ALT, alanine aminotransferase; ASC, asymptomatic carriers; AST, aspartate aminotransferase; CHB, chronic hepatitis B; CHE, cholinesterase; CRP, C-reactive protein; GGT, γ-glutamyl transferase; HBeAg, hepatitis B virus e antigen; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; HBV-ACLF, hepatitis B virus related acute-on-chronic liver failure; HBV DNA, hepatitis B virus DNA; HBV-LC, hepatitis B virus related liver cirrhosis; Hgb, hemoglobin; INR, international normalized ratio; MELD score, model end-stage liver disease; NC, normal control; Neut, neutrophil count; PLT, platelet; PT, prothrombin time.
2.2 Hematologic, biochemical, and virological parameters
Albumin (ALB), TBil, ALT, and aspartate aminotransferase, cholinesterase levels, white blood cell count, neutrophil percentage and count, hemoglobin level, prothrombin time, INR, C-reactive protein (CRP), procalcitonin, HBsAg, HBV e antigen (HBeAg), and hepatitis B DNA expression levels were recorded at the time of admission. Further, the end-stage liver disease (MELD) and Child-Pugh scores of the participants were calculated as previously described.16
2.3 Flow cytometry
Consistent with a previous study, peripheral blood mononuclear cells (PBMCs) were extracted via Ficoll-Hypaque density gradient centrifugation by Cedarlane Laboratories.17 Notably, PBMCs were labeled with the following monoclonal antibodies: FITC anti-human CCR5, PE anti-human B, and T lymphocyte attenuator (BTLA), ECD anti-human CD4, PE-Cy5.5 anti-human CD127, PE-Cy7 anti-human CD64, APC anti-human CD7, APC-AF700 anti-human CD3, APC-AF750 anti-human CD57, BV410 anti-human CD25, and KO anti-human CD45. All the antibodies were purchased from Beckman Coulter. Further, data collection and analysis were performed using the Navios program (Beckman Coulter).
2.4 In vitro induction assay and annexin V-apoptosis assay
PBMCs from healthy donors at a concentration of 1 × 106 cells per ml were cultured for 3 days in a complete medium (RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 g/ml streptomycin; Life Technologies) with varying concentrations of recombinant human (rh) interleukin (rhIL-22) (10−50 ng/ml) (PeproTech). Every other day, the medium and cytokines were changed. Finally, cells were collected and labeled with BV510 anti-human CD4, FITC anti-human CD7, APC-Cy7 anti-human CD3, APC anti-human CD57, PE anti-human annexin V, and Percp-cy5.5 anti-human PI (BD Bioscience), and the frequency of CD3+CD4−CD7CD57− T cells as well as apoptosis rates were detected via flow cytometry analysis (Navios; Beckman Coulter).
2.5 Analysis of cytokine production
CD7+CD57,+ CD7+CD57,− CD7−CD57,+ and CD7−CD57− cells were sorted from CD3+CD4− T cells using the MoFlo XDP cell sorter (Beckman Coulter) at a purity of >95%. After that, the cells were stimulated in 96-well plates using phorbol 12-myristate-13-acetate (PMA)/ionomycin/Brefeldin A (BFA) (Sigma-Aldrich) for 5 h. Afterward, the Cytofix/Cytoperm Plus kit was used to fix and permeabilize the cells (BD Biosciences). Additionally, intracellular antibodies (BV421 anti-human IFN-γ, PE-Cy7 anti-human TNF-α, Percp-Cy5.5 anti-human granzyme A, and PE anti-human perforin) were then used to stain the cells. These antibodies were purchased from BD Biosciences.
2.6 Statistical analysis
GraphPad Prism 8.0 (GraphPad Software Inc.) was used for statistical analysis in this study. The results obtained were presented as mean ± SEM. Further, the Wilcoxon test, Mann−Whitney U test, Kruskal−Wallis H test, and Dunn's multiple comparison test were performed to compare data across or among groups, while Spearman's correlation test was performed to determine correlations.
3 RESULTS
3.1 Frequency of CD3+CD4−CD7+CD57− T cells decreased in patients with end-stage liver disease
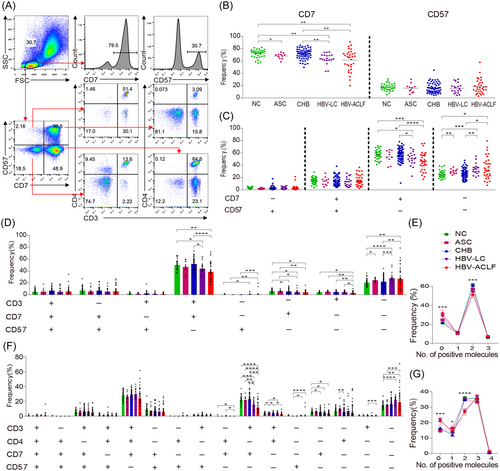
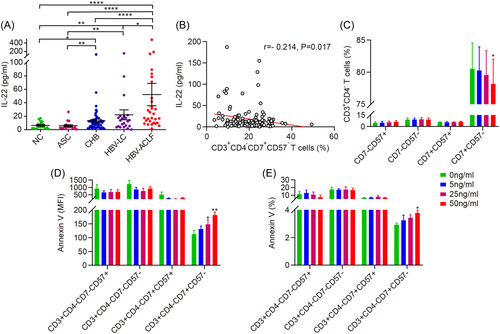
We examined the expression of CD7 and CD57 in PBMCs obtained from patients with HBV infection and individuals in the NC group to determine which T cell subsets are involved in the pathogenesis of HBV infection (Figure 1A). First, we noted that the NC, ASC, CHB, HBV-LC, and HBV-ACLF groups showed comparable CD57 expression levels in PBMCs, whereas CD7 expression was significantly lower for the patients in the HBV-LC and HBV-ACLF groups than for patients in the CHB group and the controls. Further, PBMCs from patients in the HBV-LC and HBV-ACLF groups showed no discernible changes in CD7 expression (Figure 1B). Second, we identified variations in the frequency of immune cells among the five groups. These variations were further classified into four categories based on the expression of CD7 and CD57. Compared with the CD7 and CD57 expression levels corresponding to the NC and CHB groups, the frequency of CD7+CD57− cells significantly decreased for the HBV-LC and HBV-ACLF groups, whereas the frequency of CD7−CD57− cells markedly increased. No significant differences were observed between the groups regarding the frequencies of CD7−CD57+ and CD7+CD57+ cells (Figure 1C). Third, to further confirm the downregulation of CD7+CD57− T cells, their frequency in CD3+ T cells was determined. Thus, we observed that the frequency of CD3+CD7+CD57−cells was significantly lower for the HBV-LC and HBV-ACLF groups than for the NC and CHB groups (Figure 1D). Furthermore, we determined whether CD7+CD57−cells were decreased in CD4+ or CD8+ T cells in patients in the HBV-LC and HBV-ACLF groups. Surprisingly, the frequency of CD7+CD57− cells decreased only in CD3+CD4− (CD8+) T cells and not in CD3+CD4+ (CD4+) T cells in patients in the HBV-LC and HBV-ACLF groups (Figure 1F). Significant differences in the frequency of CD4+CD8+ double-positive T cells were also observed among the five groups (Figure 1E,G).
3.2 Frequency of CD3+CD4−CD7+CD57− T cells negatively correlated with disease progression, prognosis, and complications in patients with end-stage liver disease
To investigate whether the frequency of CD3+CD4−CD7+CD57− T cells was associated with disease severity, we assessed the relationship between CD3+CD4−CD7+CD57− T cells and clinical parameters. Thus, we observed that the frequency of CD3+CD4−CD7+CD57− T cells was negatively correlated with disease severity (TBil, prothrombin time, INR, immunoglobulin G, Child-Pugh, and MELD scores) and age but positively correlated with the compensatory indices of liver function (cholinesterase and ALB), inflammatory indices (neutrophil percentage and count, and CRP), and HBV replication (HBsAg, HBeAg, and HBV DNA) (Figure 2A,B and Supporting Information: Figure S1B−D).
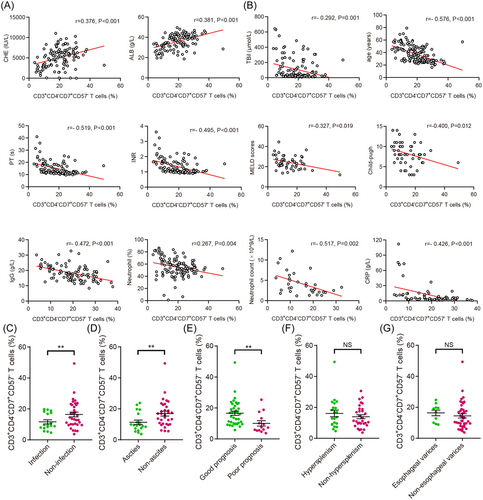
Next, to investigate the association between CD3+CD4−CD7+CD57− T cells and prognosis, 51 patients with end-stage liver disease were separated into two groups: those with good prognosis (n = 37) and those with poor prognosis (n = 14). In a previous study, our team described the specific characteristics of the two groups.16 The good prognosis group included patients whose liver hematology tests gradually improved and who were still alive within 6 months, while the poor prognosis group included patients with progressive liver deterioration or patients who had liver transplantation or died within 6 months. As expected, the frequency of CD3+CD4−CD7+CD57− T cells was higher in the good prognosis group than in the poor prognosis group (Figure 2E). Further, we examined the distribution of CD3+CD4−CD7+CD57− T cells concerning complications in patients with end-stage liver disease. Thus, we observed that the frequency of CD3+CD4−CD7+CD57− T cells was lower in patients with ascites and infection complications than that in those without these complications (Figure 2C,D), while there was no significant difference between patients with or without hypersplenism or esophageal varices in this regard (Figure 2F,G). Moreover, after comprehensive treatment, CD3+CD4−CD7+CD57− T cells and the level of TBil in patients with HBV-ACLF gradually decreased synchronously (Supporting Information: Figure S1A).
3.3 CD3+CD4−CD7+CD57− T cells displayed unique phenotypes
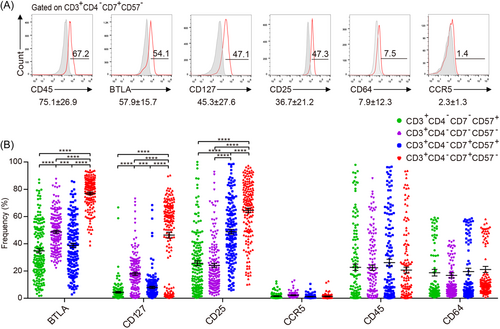
To characterize the phenotype of CD3+CD4−CD7+CD57− T cells, the expression levels of CD45, BTLA, CD127 (T cell survival), CD25 (activation), CD64, and CCR5 in CD3+CD4−CD7+CD57− T cells was analyzed. The results indicated the dominance of CD45, followed by BTLA, CD127, CD25, CD64, and then CCR5 (Figure 3A). Next, we compared the expression profiles of four CD3+CD4− T cell populations: CD7−CD57,+ CD7−CD57,− CD7+CD57,+ and CD7+CD57− T cells. Among all the CD3+CD4− T cells subsets, CD7+CD57− T cells showed the highest BTLA, CD127, and CD25 expression levels (Figure 3B).
3.4 Production of functional cytokines was decreased in CD3+CD4−CD7+CD57− T cells
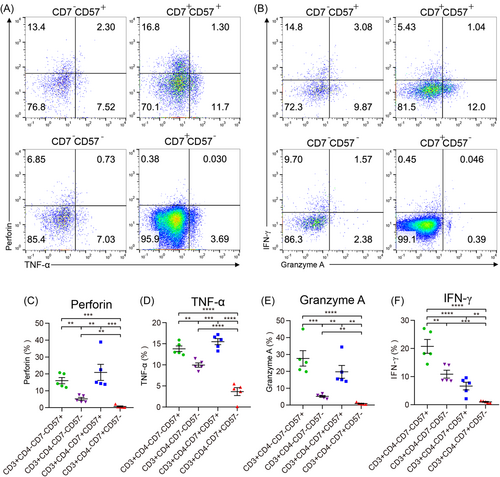
Using the markers CD7 and CD57, CD7+CD57,− CD7+CD57,+ CD7−CD57,− and CD7−CD57+ cell populations were identified and sorted from CD3+CD4− T cells via flow cytometry (Figure 4A,B). To further explore the functional characteristics of the CD3+CD4−CD7+CD57− T cells, the cytokine production levels induced by PMA/ionomycin/BFA for the different cells were compared. The results showed that the levels of IFN-γ, TNF-α, granzyme A, and perforin in CD7+CD57− T cell subsets were the lowest among all the subgroups of CD3+CD4− T cells (Figure 4C−F).
3.5 IL-22 inhibited the expansion of CD3+CD4−CD7+CD57− T cells via apoptosis
To provide a mechanistic explanation for the decreased frequency of CD3+CD4−CD7+CD57− T cells, we investigated the role of circulating cytokines. Our results indicated that IL-22 was markedly increased in the HBV-LC and HBV-ACLF groups compared with the NC, ASC, and CHB groups (Figure 5A). Additionally, we observed that IL-22 levels were negatively correlated with the frequency of CD3+CD4−CD7+CD57− T cells (Figure 5B). Therefore, we used rhIL-22 to stimulate PBMCs obtained from individuals in the NC group in vitro and observed that the frequency of CD3+CD4−CD7+CD57− T cells decreased dose-dependent (Figure 5C). Further, the frequency of annexin V (or mean fluorescence intensity) increased dose-dependent (Figure 5D,E).
4 DISCUSSION
Two cell surface molecules involved in developing the operational phases of T lymphocytes are CD7 and CD57. In this study, we aimed to clarify the function of these two surface markers, which offer the possibility to distinguish CD8+ T cells during HBV infection and can serve as new prognostic tools. Thus, we observed a decrease in the frequency of CD8+ T cells that coexpress CD7 and lack CD57 (CD3+CD4−CD7+CD57− T cells) in patients with HBV-LC and HBV-ACLF. A negative correlation was also observed between the frequency of CD3+CD4−CD7+CD57− T cells and disease progression, prognosis, and complications in these patients, indicating that CD3+CD4−CD7+CD57− T cells function as a protective factor for preventing the liver disease from progressing to an end-stage.
Notably, it is yet unknown why the frequency of CD3+CD4−CD7+CD57− T cells declines in patients with end-stage liver disease. The aging immune system, especially in old age, may gradually deteriorate, possibly accounting for the decline in the count of these cells. Our findings also consistently showed a negative correlation between age and the frequency of CD3+CD4−CD7+CD57− T cells (Supporting Information: Figure S1). However, it is known that T cells acquire a terminally differentiated state with age, which may cause lineage bias rather than a decrease in their number.18 In addition, the age of patients with end-stage liver disease included in this study was higher than that of patients with CHB and ACS; this might have led to the perception that CD3+CD4−CD7+CD57− T cells were negatively correlated with age. The likelihood of a decline of CD3+CD4−CD7+CD57− T cells resulting from HBV infection was not considered because CD3+CD4−CD7+CD57− T cells were positively associated with HBV replication.
Patients with end-stage liver disease frequently exhibit systemic inflammatory response syndrome, particularly patients with ACLF.19 Additionally, IL-22 exhibits a double-edged sword-like behavior as it reduces liver inflammation and protects against ACLF in the early stage while promoting the transition of CHB to ACLF as a proinflammatory factor.19-22 In this study, IL-22 levels were significantly elevated in patients with HBV-ACLF and showed a negative correlation with the frequency of CD3+CD4−CD7+CD57− T cells. Therefore, we hypothesized that IL-22 might inhibit CD3+CD4−CD7+CD57− T cell expansion; this was confirmed by the decreased CD3+CD4−CD7+CD57− T cells after stimulation with IL-22 in a dose-dependent manner. This decrease in the frequency of CD3+CD4−CD7+CD57− T cells following IL-22 stimulation could be primarily attributed to the increased apoptosis of CD3+CD4−CD7+CD57− T cells promoted by IL-22. Therefore, blocking the IL-22 signaling pathway and restoring the number of CD3+CD4−CD7+CD57− T cells might be an effective immunotherapy strategy against ACLF; this needs to be clarified in future studies.
Previous studies have shown that CD8+CD57+ T cells express high programmed cell death-1 (PD-1) and exhibit enhanced cytotoxic potency and impaired proliferative capability.6 In this study, CD3+CD4−CD7+CD57− T cells expressed high levels of BTLA, a coinhibitory molecule similar to PD-1.23 Therefore, consistent with their functional phenotype, CD3+CD4−CD7+CD57− T cells expressed less functional cytokines, including IFN-γ, TNF-α, granzyme A, and perforin. Thus, we assumed that in contrast to cytotoxic CD8+ T cells, CD3+CD4− (CD8+) CD7+CD57− T cells exhibit a nonfunctional phenotype, thereby inactivating the immune-inflammatory response and alleviating liver injury.
The study had a few limitations: (1) The number of patients with chronic HBV infection was not sufficiently large, particularly the number of patients with ASC; (2) No IL-22 neutralization or blocking assays were performed to demonstrate its proinflammatory effects; and (3) The protective roles of CD3+CD4−CD7+CD57− T cells in the liver were not examined using animal models.
5 CONCLUSIONS
This study revealed that the frequency of a subset of liver-protective cells, particularly CD3+CD4−CD7+CD57− T cells, was considerably reduced in individuals with end-stage liver disease, especially in those with HBV-ACLF. Further, IL-22 also decreased the frequency of these CD3+CD4−CD7+CD57− T cells by promoting their apoptosis. Thus, CD7 and CD57 expression in CD8+ T cells have prognostic significance in patients with end-stage liver disease.
AUTHOR CONTRIBUTIONS
Study concept and design: Xueping Yu, Yijuan Zheng, Dawu Zeng, Jian Sun, Jiming Zhang, Chunfu Zheng, Yijuan Zheng, and Zhijun Su. Data acquisition, analysis, and interpretation: Xueping Yu, Dawu Zeng, Yijuan Zheng, and Jian Sun. Material support: Milong Su, Huatang Zhang, Minhui Zheng, Wenwu Lin, Richeng Mao, and Yijuan Zheng. Drafting of the manuscript: Xueping Yu, Yijuan Zheng, and Jian Sun. Critical manuscript revision for important intellectual content: Xueping Yu, Richeng Mao, Jiming Zhang, Chunfu Zheng, and Zhijun Su. Obtained funding: Xueping Yu, Richeng Mao, and Jiming Zhang.
ACKNOWLEDGMENTS
The authors are grateful to the Department of Infectious Diseases, Fujian Medical University Affiliated First Quanzhou Hospital, for the assistance provided in clinical specimen collection and to all staff at the Life Sciences and Chemistry College, Minnan Science and Technology University. This study was supported by the National Natural Science Foundation of China (grant numbers 81400625, 81670528 and 81672007), the Natural Science Foundation of Fujian Province (grant numbers 2019J01593 and 2019Y9048), the Young and Middle-Aged Key Talent Training Project of Fujian Province (grant number 2020GGA076), and the High-level Talent Innovation Project of Quanzhou (grant number 2018C067R).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of Fujian Medical University Affiliated First Quanzhou hospital.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Journal of medical virology at https://pan.baidu.com/s/1eNJKHujJ8AhlB-WuqxCYwA?pwd=rsnf, reference number rsnf.




