Association of viral load with TRAIL, IP-10, CRP biomarker signature and disease severity in children with respiratory tract infection or fever without source: A prospective, multicentre cohort study
Cihan Papan, Alberto Argentiero, Susanna Esposito and Tobias Tenenbaum contributed equally to this study.
The material was presented in part at the 2019 meeting of the European Society for Paediatric Infectious Diseases in Ljubljana, Slovenia (May 6–11, 2019).
Abstract
Background
To investigate the association of viral load (VL) with (i) tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), interferon gamma-induced protein-10, C-reactive protein, and a combinatorial score (BV score), and (ii) clinical severity.
Study Design
In this prospective, multicentre cohort substudy, children with respiratory tract infection or fever without source were enrolled. VL for influenza virus, rhinovirus, respiratory syncytial virus, and adenovirus was measured from nasopharyngeal swabs. The reference standard diagnosis was established based on expert panel adjudication.
Results
Of 1140 recruited patients, 333 had a virus monodetection. VL for the aggregated data set correlated with TRAIL and IP-10 levels, with the length of oxygen therapy, and inversely with the BV score. At a single viral level, only the influenza VL yielded a correlation with TRAIL, IP-10 levels, and the BV score. Children with a viral reference standard diagnosis had significantly higher VL than those with bacterial infection (p = 0.0005). Low TRAIL (incidence rate ratio [IRR] 0.6, 95% confidence interval [CI] 0.39–0.91) and young age (IRR 0.62, 95% CI 0.49–0.79) were associated with a longer hospital stay, while young age (IRR 0.33, 95% CI 0.18–0.61), low TRAIL (IRR 0.25, 95% CI 0.08–0.76), and high VL (IRR 1.16, 95% CI 1.00–1.33) were predictive of longer oxygen therapy.
Conclusion
These findings indicate that VL correlates with biomarkers and may serve as a complementary tool pertaining to disease severity.
1 BACKGROUND
Correctly establishing infectious disease aetiology remains a major challenge, especially for respiratory tract infections (RTI), which results in a significant tendency to treat most patients with antibiotics.1 This overuse is a major driver of the rising antimicrobial resistance rates observed during the past decades and projected to continue.2
Viral detection technologies are often applied to support aetiology determination. However, a known limitation of viral detection technologies, including viral polymerase chain reaction (PCR), is that bacterial coinfection is not ruled out. Several novel host biomarkers to aid the clinician in differentiating between bacterial and viral infection have recently been discovered and described. Notably, a combinatorial score based on three host proteins, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), interferon gamma-induced protein-10 (IP-10), and C-reactive protein (CRP) has shown superior diagnostic accuracy compared with routine markers of inflammation in patients with RTI or fever without source (FWS).3-6 The high performance of this score (combinatorial score [BV score]) is likely due to the fact that it encompasses two virally induced biomarkers, TRAIL, and IP-10, in addition to a predominantly bacterially induced biomarker, CRP, thereby distilling information from complementary biological pathways. A useful feature of the BV score is that it yields bacterial scores in cases of bacterial and viral coinfection. In this way, viral detection and host biomarkers can offer nonredundant information for clinicians.
Besides aetiology, another pivotal clinical question concerns disease severity. Previous studies that move beyond the qualitative approach, that is, the presence or absence of a virus, and take into account the viral load (VL) have yielded conflicting results on its relationship with severity.7-12
Here, we sought to investigate in children with a viral monodetection (i) the association of VL with TRAIL, IP-10, CRP, and the BV score and (ii) the association of VL with indicators of clinical severity.
2 STUDY DESIGN
2.1 Setting and population
This is a subanalysis of the AutoPilot-Dx Study, which was a multicentre, prospective, cohort study run at two University Hospitals in Germany and Italy.13 Patients aged 90 days or older presenting consecutively to the children's hospital were eligible when the following inclusion criteria were met: clinical suspicion of RTI or FWS; body temperature ≥38.0°C; and a history of present illness ≤7 days. Exclusion criteria were: febrile episode during the previous 14 days; antibacterial therapy for more than 48 h; (suspected) HIV, HBV, or HCV infection; primary immunodeficiency; active malignancy; immunosuppressive or immunomodulatory treatment; severe psychomotor retardation; and severe congenital metabolic disorders.
Only children with a viral monodetection were included in this sub-study. The study was approved by the respective Ethical committees (2016-410M-MA-§ 23b MPG; 10042/17/AV/DM CEAS Umbria). All patients' parents gave informed consent before study inclusion. Reporting of this substudy adhered to the STROBE guidelines (Supporting Information: Table 1).
2.2 Study procedures
Upon recruitment, a nasopharyngeal swab was taken from each participant with a flexible flocked swab and placed in Universal Transport Medium (Copan). The samples were vortexed, split into two aliquots, and stored at −80°C. One aliquot was used for semiquantitative/qualitative multiplex PCR for the detection of viruses and bacteria (Allplex™ Respiratory Panel; Seegene). Pathogens covered within this test are: adenovirus, coronavirus 229E, coronavirus NL63, coronavirus OC43, parainfluenza virus 1, parainfluenza virus 2, parainfluenza virus 3, parainfluenza virus 4, rhinovirus, influenza A virus, influenza B virus, influenza A-H1 virus, influenza A-H1pdm09 virus, influenza A-H3 virus, respiratory syncytial virus A, respiratory syncytial virus B, human metapneumovirus, bocavirus, enterovirus, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Haemophilus influenzae, Streptococcus pneumoniae, Bordetella pertussis, and Bordetella parapertussis. The second aliquot was subjected to VL measurement when one of the following viruses was detected in the multiplex PCR: influenza virus (FLU), respiratory syncytial virus (RSV), adenovirus (AdV), or rhinovirus (HRV).
2.3 VL measurements
All eligible specimens were submitted to the Institute of Virology of the University Hospital Düsseldorf and evaluated for RSV (subtype A and B), HRV (species A, B, and C), FLU types A and B, and AdV using a quantitative real-time reverse-transcription PCR as described elsewhere.14 Quantitation for virus-genomes was performed using known concentrations of plasmids containing the target regions of the viruses. A standard graph of the cycle threshold (CT) values obtained from serial dilutions of the standard was constructed by the software, the CT values of the unknown samples were plotted on the standard curves and the number of genomes was calculated. VLs were indicated in genome equivalents per milliliter (geq/ml).
2.4 BV score
BV score measurements were performed as described previously13 on serum samples according to the instructions of the manufacturer (ImmunoXpert™; MeMed). All staff performing the measurements were blinded to the clinical diagnosis and the reference standard outcome. The signature combines the serum concentrations of TRAIL, IP-10, and CRP using a locked computational algorithm, which generates a score from 0 to 100. The predetermined score cut-offs provided by the manufacturer are as follows: <35 is classified as viral infection, >65 is classified as bacterial infection, and 35–65 is deemed as equivocal.
2.5 Reference standard outcome
In the absence of a “gold standard” for determining bacterial and viral aetiologies, we employed an expert panel approach, whereby three independent adjudicators, paediatricians with >10 years of clinical experience, were asked to assign one of the following diagnoses to a case: bacterial (including bacterial/viral coinfection), viral, indeterminate, or noninfectious aetiology. To prevent bias, adjudicators were blinded to each other and to the BV score, yet had access to all remaining patient data (including multiplex PCR). Cases not assigned the same diagnosis by the three adjudicators were regarded as indeterminate.
2.6 Statistical analysis
R Studio (Version 1.3.1093) and Prism 9.0.0 (GraphPad) were used for statistical analyses. Characteristics between the two groups were compared using the Mann–Whitney U test or t test for continuous variables and χ2 or Fisher's exact test for categorical data. Comparisons between three groups were performed with one-way analysis of variance or the Kruskal–Wallis test. Spearman rank correlation was used to test the correlation between VL and TRAIL, IP-10, CRP, BV Score, and other variables. Kaplan–Meier analysis was performed to investigate the impact of VL on the probability of oxygen therapy. Furthermore, we assessed the potential association between the biomarkers and VL by performing linear regression analyses. Additionally, we developed a quasi-Poisson regression model for the outcome variables length of stay and length of oxygen therapy as markers of disease severity. The predetermined covariables were age, sex, length of symptoms before presentation, the reference standard diagnosis, antibiotic therapy, and biomarker levels. Logarithmic transformation was performed for predictor variables with non-normal distribution. When comparing VL with TRAIL and IP-10 levels, cut-offs of 80 and 400 pg/ml were employed, respectively. For group comparisons depending on the BV Score outcome, the predetermined, aforementioned cut-offs provided by the manufacturer were used. The statistical significance level was set at 0.05.
3 RESULTS
3.1 Patient characteristics
In total, 1140 patients were recruited between February 2017 and December 2018, of whom 1077 met the eligibility criteria of the AutoPilot-Dx study. Of these, 333 had a monodetection of either FLU, RSV, AdV, or HRV. Table 1 depicts the patient characteristics of this substudy population with single viral detections. Of these, 232 (69.7%) were assigned by the adjudication process as viral infections, 20 (6.0%) as bacterial infections, and the remaining 81 (24.3%) considered indeterminate cases. The majority of children, 254 (76.3%) were diagnosed with a respiratory tract infection. The mean (SD) age of the cohort was 3.38 (3.26) years; 42.3% were female. Antibiotics were prescribed to 148/333 (44.4%) of the children. The most frequently monodetected virus was FLU (107/333; 32.1%), followed by HRV (95/333; 28.5%), RSV (75/333; 22.5%), and AdV (56/333; 16.8%). VLs were highest for RSV, followed by Flu and ADV, while children with HRV detection had the lowest VLs (Table 1).
| FLU (n = 107) | HRV (n = 95) | RSV (n = 75) | AdV (n = 56) | All (n = 333) | |
|---|---|---|---|---|---|
| Age in years (mean, SD) | 4.55 (3.59) | 3.56 (3.47) | 1.68 (1.36) | 3.12 (3.11) | 3.38 (3.26) |
| Gender, female, n (%) | 48 (44.9) | 39 (41.1) | 33 (44.0) | 21 (37.5) | 141 (42.3) |
| Max. temperature in °C (mean, SD) | 39.4 (0.71) | 38.9 (0.78) | 39.2 (0.74) | 39.6 (0.82) | 39.3 (0.79) |
| Days since symptom onset (mean, SD) | 3.0 (2.12) | 2.15 (1.58) | 3.2 (1.6) | 2.9 (1.8) | 2.79 (1.85) |
| Days since fever onset (mean, SD) | 2.8 (2.11) | 1.55 (1.43) | 2.5 (1.6) | 2.7 (1.8) | 2.37 (1.83) |
| Antibiotics initiated, n (%) | 32 (29.9) | 52 (54.7) | 30 (40) | 34 (60.7) | 148 (44.4) |
| Hospitalized, n (%) | 68 (63.4) | 81 (85.3) | 62 (82.7) | 32 (57.1) | 243 (73.0) |
| Hospital length of stay, days (median, IQR) | 3 (0–4) | 4 (2–6.5) | 4 (2.5–7) | 2 (0–4) | 3 (0–5) |
| Oxygen therapy, n (%) | 8 (7.5) | 29 (30.5) | 41 (54.7) | 1 (1.8) | 79 (23.7) |
| Intensive care, n (%) | 0 | 1 (1.1) | 0 | 0 | 1 (0.3) |
| Viral load, geq/ml (median, IQR) | 146,626.6 (17,440.4–1,215,538.1) | 2,557.8 (306.7–37,717.9) | 2,207,811.1 (544,089–8,799,859) | 112,409.8 (8369.7–3,068,339.2) | 113,356.7 (3091.1–1,471,116.9) |
| BV score (median, IQR) | 2 (0–13.5) | 49 (8.5–86.5) | 12 (4–27) | 38.5 (8.8–66.5) | 12 (2–50) |
| TRAIL (median, IQR) | 147.4 (90.5–250.6) | 61.2 (41.6–103.2) | 89.3 (69.2–127.5) | 82.5 (63.1–116) | 94.4 (62.8–152.1) |
| IP-10 (median, IQR) | 688.9 (426–1153.1) | 223.53 (132.30–433.1) | 393.8 (250.4–540.1) | 375.9 (303.8–548.2) | 411.8 (248.4–688.9) |
| CRP (median, IQR) | 8.8 (3.5–25.4) | 34 (13–84.1) | 10.5 (5.6–30.4) | 49.2 (31.9–78.1) | 17 (5.8–49.4) |
| Reference standard outcome viral, n (%) | 92 (86.0) | 51 (53.7) | 58 (77.3) | 31 (55.4) | 232 (69.7) |
| Reference standard outcome indeterminate, n (%) | 13 (12.6) | 34 (35.8) | 13 (17.3) | 21 (37.5) | 81 (24.3) |
| Reference standard outcome bacterial, n (%) | 2 (1.9) | 10 (10.5) | 4 (5.3) | 4 (7.1) | 20 (6.0) |
| Diagnosis group | |||||
| Respiratory tract infection, n (%) | 55 (51.4) | 81 (85.3) | 72 (96) | 46 (82.1) | 254 (76.3) |
| Acute otitis media, n (%) | 6 (5.6) | 5 (5.3) | 1 (1.3) | 3 (5.4) | 15 (4.5) |
| Pneumonia, n (%) | 10 (9.3) | 26 (27.4) | 33 (44) | 9 (16.1) | 78 (23.4) |
| Fever without source, n (%) | 52 (48.6) | 14 (14.7) | 3 (4) | 10 (17.9) | 79 (23.7) |
- Abbreviations: AdV, adenovirus; CRP, c-reactive protein; FLU, influenza virus; HRV, rhinovirus; IP-10, interferon gamma-induced protein-10; IQR, interquartile range; RSV, respiratory syncytial virus; SD, standard deviation; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
3.2 VL correlation with host biomarkers
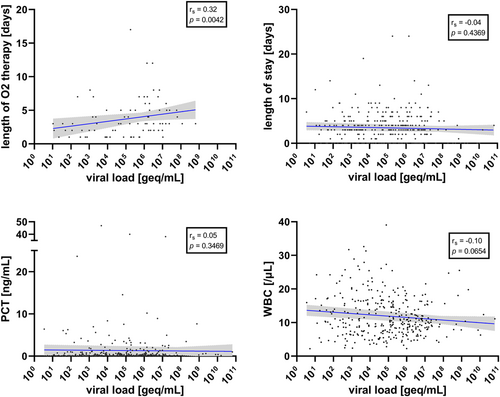
Considering the four viruses together, VL was significantly positively correlated with TRAIL (Spearman coefficient rs = 0.16, 95% confidence interval, CI, 0.05–0.27; p = 0.003) and IP-10 levels (rs = 0.17, 95% CI 0.06–0.28; p = 0.002), and significantly negatively correlated with BV score (rs = −0.17, 95% CI −0.27 to −0.06; p = 0.002) (Figure 1). In contrast, VL was not significantly correlated with CRP (rs = −0.05, 95% CI −0.16 to 0.06; p = 0.33) or PCT (rs = 0.05, 95% CI −0.06 to 0.16; p = 0.347) or WBC (rs = −0.10, 95% CI −0.21 to 0.01; p = 0.065) (Figures 1 and 2).
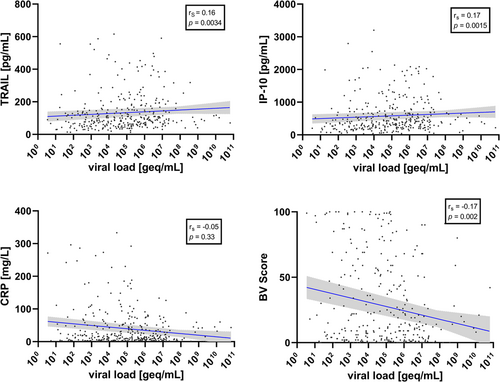
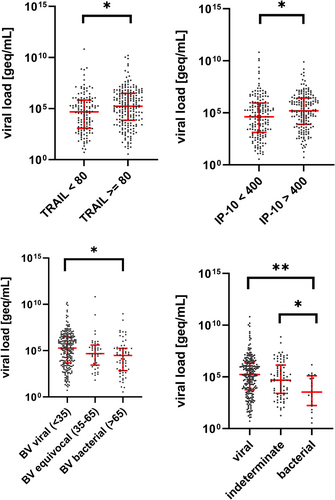
Accordingly, VLs were significantly higher in children with TRAIL > 80 pg/ml (p = 0.007), with IP-10 > 400 pg/ml (p = 0.005) and with a viral BV score (<35) as compared with a bacterial score (>65; p = 0.0013) (Figure 3). At the single virus level, only FLU VL was significantly correlated with TRAIL (rs = 0.33, 95% CI 0.15–0.49; p = 0.0005), IP-10 (rs = 0.30, 95% CI 0.12–0.46; p = 0.0017), and BV score (rs = −0.30, 95% CI −0.46 to −0.12); p = 0.0018) (Supporting Information: Figures 1–3). For HRV, RSV, and AdV, no significant correlation was found between VL and levels of TRAIL, IP-10, or the BV score (Supporting Information: Figures 1–3). Univariate linear regression analyses yielded significant associations between the outcome variable VL and the biomarkers TRAIL and IP-10, respectively (Table 2). However, none of the biomarkers showed an association in the multivariable logistic regression (Table 2).
| Variable | Estimate in univariate regression (95% CI) | p Value | Estimate in multiple regression (95% CI) | p Value |
|---|---|---|---|---|
| TRAIL | 0.92 (0.29 to 1.55) | 0.004 | 0.67 (−0.20 to 1.54) | 0.13 |
| IP-10 | 0.74 (0.21 to 1.27) | 0.007 | 0.42 (−0.25 to 1.08) | 0.22 |
| CRP | −0.16 (−0.51 to 0.19) | 0.37 | 0.05 (−0.34 to 0.43) | 0.82 |
- Abbreviations: CRP, C-reactive protein; CI, confidence interval; IP-10, interferon gamma-induced protein-10; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
3.3 VL association with clinical parameters and disease severity
VL was not significantly correlated with length of hospital stay (rs = −0.04, 95% CI −0.15 to −0.07; p = 0.437) (Figure 2). Moreover, there was no difference in VL levels between hospitalized and outpatient children (median 108,089 vs. 121,048 geq/ml; p = 0.282), children with or without antibiotic treatment (median 71,831 vs. 136,830 geq/ml; p = 0.710); children with or without oxygen therapy (median 326,351 vs. 77,784 geq/ml; p = 0.098). However, for the subgroup of patients receiving oxygen therapy, the length of oxygen therapy significantly correlated with VL. In addition, using a cut-off of 105 geq/ml, children with higher VLs were under oxygen therapy for a longer period (log-rank test p = 0.006) (Figure 4).
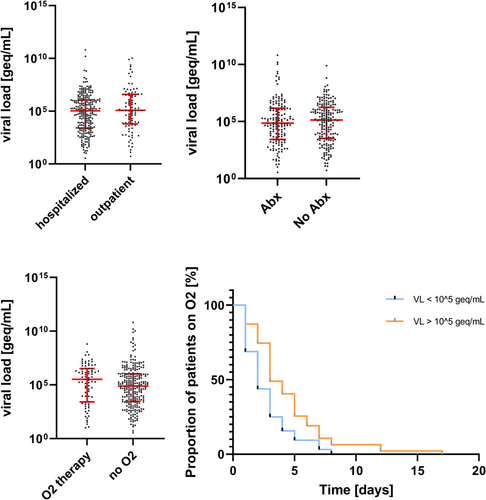
In a quasi-Poisson regression model with hospital length of stay as the outcome variable, TRAIL (incidence rate ratio, IRR, 0.60, 95% CI 0.39–0.91, p = 0.016) and patient age (IRR 0.62, 95% CI 0.49–0.79, p < 0.001) showed a negative association, alongside a reference standard diagnosis of viral or indeterminate (IRR 0.54 and 0.63, 95% CI 0.34–0.87 and 0.44–0.91, p = 0.011 and 0.013). Of note, VL was not predictive of length of stay (Table 3a).
| Variable | Incidence rate ratio | 95% confidence interval | p Value |
|---|---|---|---|
| (a) Outcome variable: length of stay | |||
| Viral load | 0.98 | 0.93–1.04 | 0.592 |
| TRAIL | 0.60 | 0.39–0.91 | 0.016 |
| IP-10 | 1.19 | 0.85–1.68 | 0.321 |
| CRP | 1.07 | 0.84–1.38 | 0.586 |
| Age | 0.62 | 0.49–0.79 | <0.001 |
| Male sex | 0.91 | 0.75–1.10 | 0.324 |
| Procalcitonin | 1.05 | 0.84–1.30 | 0.669 |
| White blood count | 0.98 | 0.58–1.65 | 0.925 |
| Duration of symptoms | 0.97 | 0.92–1.03 | 0.337 |
| Antibiotic therapy | 1.16 | 0.89–1.50 | 0.264 |
| Viral ref. standard | 0.54 | 0.34–0.87 | 0.011 |
| Indeterminate ref. standard | 0.63 | 0.44–0.91 | 0.013 |
| (b) Outcome variable: length of oxygen therapy | |||
| Viral load | 1.16 | 1.00–1.33 | 0.045 |
| TRAIL | 0.25 | 0.08–0.76 | 0.017 |
| IP-10 | 1.67 | 0.63–4.65 | 0.316 |
| CRP | 1.20 | 0.64–2.31 | 0.583 |
| Age | 0.33 | 0.18–0.61 | <0.001 |
| Male sex | 0.78 | 0.48–1.27 | 0.319 |
| Procalcitonin | 0.58 | 0.30–1.05 | 0.080 |
| White blood count | 0.31 | 0.08–1.15 | 0.079 |
| Duration of symptoms | 1.03 | 0.90–1.17 | 0.682 |
| Antibiotic therapy | 0.69 | 0.34–1.33 | 0.286 |
| Viral ref. standard | 0.41 | 0.11–1.74 | 0.208 |
| Indeterminate ref. standard | 0.62 | 0.21–2.13 | 0.417 |
- Note: Significant p values are indicated in bold.
- Abbreviations: CRP, C-reactive protein; IP-10, interferon gamma-induced protein-10; Ref. standard, reference standard; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
In contrast, VL was associated with length of oxygen therapy (IRR 1.16, 95% CI 1.00–1.33, p = 0.045), while TRAIL and patient age were similarly predictive for this outcome variable (Table 3b).
3.4 The segregation of VL per reference standard outcome
According to the reference standard outcome, VLs were significantly higher in children with a viral infection compared with bacterial infections (p < 0.0005). We further analyzed these findings on single viral levels. We found that VLs for all four viruses showed a trend for being higher in those with viral reference standard diagnosis than in those with a bacterial reference standard outcome, although statistical significance was not attained (Supporting Information: Table 2 and Figure 4).
4 DISCUSSION
Here, we show that VLs from nasopharyngeal swabs of children with either FLU, HRV, RSV, or AdV detection correlate with TRAIL, IP-10, and inversely with the combinatorial BV Score. Furthermore, we demonstrate the association of VLs with the length of oxygen therapy. In addition, we also showed that VLs were significantly higher in children with a viral infection as per reference standard outcome, compared with bacterial (co-)infections.
To the best of our knowledge, this is the first study to date to investigate the correlation between VLs in children with respiratory viruses and TRAIL, IP-10, CRP, and the BV score. Previously, several groups had shown a correlation between VL and clinical severity,15-17 while in other studies, these findings could not be replicated.18, 19 Wishaupt et al.20 showed that the VL in patients infected with RSV correlated with the odds of hospital admission. Esposito et al.21 found a higher need for oxygen in children with a higher VL of HRV, while Xiao et al.22 only demonstrated a higher disease severity for children below the age of 2 years. Of note, children with HRV in our cohort had the lowest VLs and the highest rate of indeterminate or bacterial reference standard diagnosis. This suggests that HRV, as reported previously,23 is not necessarily causative, but merely a remnant of a past infection. However, bacterial coinfection may be another plausible explanation for this, as well as asymptomatic infections.24 In addition, we found no correlation between VL for HRV and biomarker levels, compared with the aggregated VL data.
Yan et al.25 proved a correlation between VL and the diagnosis of bronchiolitis in children with RSV. Interestingly, other groups did not find a correlation between VL and the severity of disease in their respective studies.26, 27
Recently, this conundrum has been addressed in several distinct patient populations. Haddadin et al.8 demonstrated that, in RSV-infected children below 5 years of age, VL was an independent risk factor for hospitalization. Conversely, authors from another US study found that in children below 2 years of age with RSV, VLs were higher in outpatients than in inpatients, and higher in milder diseases than in severe cases.9 In another study from Finland focusing on outpatients with RSV, children with higher VLs had longer symptom duration.7
In our cohort, we were not able to establish an association between VL and length of hospital stay. However, this may have been influenced largely by antibiotic treatment, which was highly prevalent in all subgroups (between 30% and 60%). This was further substantiated by the predictive potential of TRAIL, but not of VL, on hospital length of stay in the quasi-Poisson regression. However, high VLs were predictive of longer oxygen therapy.
The use of TRAIL and IP-10 alongside CRP in a combinatorial score had been proposed previously.3 While it has been established that TRAIL and IP-10 are virally induced, no study to date had investigated the extent to which differential VLs correlate and cosegregate with TRAIL and IP-10 levels. Our data demonstrate the clear physiological link between the intensity of viral replication and the host immune response, exemplified by TRAIL and IP-10 levels.
There are several strengths of our study. First, we used a rigorous reference method, as described previously,28 incorporating extensive clinical data and experts from the field in a blinded adjudication process. Second, our cohort comprised children of different age groups and from two different centres and countries, substantiating that the results are generalizable. Third, including more than one virus contributed to the robustness of our data.
However, there are also limitations pertinent to this study. As the study was not designed and appropriately powered for each virus, statistical significance was not attained except in the case of Influenza. The correlation findings may have been in part influenced stronger by some of the viral subgroups than the others, as indicated by the single viral correlation results, in contrast to the aggregated data. However, differences between viruses may be plausible in light of the discrepant baseline demographics such as age and diagnosis, with FLU patients being substantially older and presenting with FWS more frequently than RSV patients. Similarly, we did not investigate viral codetections, for which conflicting results had been reported,29-32 partly explained by competitive interplays between different viruses such as RSV and HRV.33 In addition, our cohort consists of non-ICU patients, which limits the inference regarding very severe cases. Likewise, the rate of acute otitis media in our cohort of mainly hospitalized children differs substantially from previously reported outpatient children with RSV or influenza in whom acute otitis media was more frequently diagnosed, mostly in the clinical follow-up.34, 35 Lastly, comparability between studies is hampered by the fact that some authors report solely CT values, while others calculate genome equivalents.
Our findings support the potential role of using VL to inform clinicians about infectious disease severity in children. With the lessons learned from the coronavirus disease 2019 (COVID-19) pandemic,36-38 healthcare workers have become acquainted with Ct values as a surrogate for VL, which is frequently used to estimate infectiousness.39 Although the correlation of SARS-CoV-2 VL and clinical severity has been demonstrated,40-43 the role of VL in assessing potential bacterial coinfections in COVID-19 patients has not systematically been performed.
Future studies should prospectively establish clinically relevant CT or geq-thresholds for specific viruses and evaluate the impact of reporting VLs on clinical care, especially pertaining to antibiotic prescription, thereby opening novel possibilities for antimicrobial stewardship.
AUTHOR CONTRIBUTIONS
Cihan Papan and Tobias Tenenbaum conceived the study and its design, had full access to the data, and take responsibility for the integrity of the data and accuracy of the analysis. Funding acquisition was made by Cihan Papan, Tobias Tenenbaum, and Susanna Esposito. Cihan Papan, Alberto Argentiero, Ortwin Adams, Marian Porwoll, Ummaya Hakim, Edoardo Farinelli, Ilaria Testa, Maria Bruna Pasticci, Daniele Mezzetti, Katia Perruccio, Arne Simon, Johannes G. Liese, Markus Knuf, Michal Stein, Renata Yacobov, Ellen Bamberger, and Sven Schneider organized and entered data. Cihan Papan, Alberto Argentiero, Ortwin Adams, Susanna Esposito, and Tobias Tenenbaum contributed to data analyses. All authors had access to the underlying data for verification and contributed to data interpretation. Cihan Papan and Tobias Tenenbaum wrote the main draft of the manuscript. All authors contributed to the final drafting of the manuscript.
ACKNOWLEDGMENTS
We thank all physicians and nurses at the University Children's Hospital Mannheim, Heidelberg University, and the Pediatric Clinic, Università Degli Studi di Perugia, and Liat Etshtein, Niv Mastboim, Einat Moscoviz, Tahel Ilan-Bar, Asi Cohen, Einav Simon, Olga Boico, Liran Shani, Tanya M. Gottlieb, Roy Navon, Eran Barash, Kfir Oved, and Eran Eden from MeMed Diagnostics for their support. This study was supported by the 2018 Small Grant Award of the European Society for Paediatric Infectious Diseases awarded to Cihan Papan. The AutoPilot-Dx study was supported by the European Commission, Executive Agency for Small and Medium-sized Enterprises, Horizon2020-FTIPilot-2015-1 program (grant number 701088). The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Open Access funding enabled and organized by Projekt DEAL.
CONFLICTS OF INTEREST
Susanna Esposito and Tobias Tenenbaum were co-applicants together with MeMed Diagnostics on the grant European Commission, Executive Agency for Small and Medium-sized Enterprises, Horizon2020-FTIPilot-2015-1 program (grant number 701088). Cihan Papan has performed other clinical research in collaboration with MeMed funded by a grant awarded to MeMed, European Commission, Executive Agency for Small and Medium-sized Enterprises H2020-EIC-SMEInst-2018-2020-2 (grant number 88124). The remaining authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




