Are bladder washing samples suitable for investigation of HPV infection in urinary bladder? Comparison in HPV prevalence between urine and washing samples
Abstract
Although urine and bladder washing samples are commonly used for the cytological evaluation of the bladder mucosa, it has been unknown whether these samples are likely suitable to investigate human papillomavirus (HPV) prevalence in the urinary bladder. The present study aimed to elucidate the appropriateness of spontaneously voided urine or bladder washing in screening HPV infection in the urinary bladder. Urine and bladder washing samples were obtained from 201 patients who underwent transurethral bladder tumor resection. After extracting DNA from both samples, HPV-DNA was examined using a nested polymerase chain reaction with GP5+/6+ and MY09/11 primers. HPV genotyping was performed in the HPV-positive samples. In situ hybridization (ISH) was performed to observe the HPV-DNA localization in urothelial cells among cytological samples and paraffin-embedded tumor tissues in HPV-positive washing samples. HPV prevalence in urine and washing samples were 9.5% and 7.0%, respectively. High-risk HPV prevalence in urine and washing samples was 7.5% and 4.0%, respectively. The most common HPV type was HPV 16, followed by HPV 52 and HPV 18 in both samples. HPV type distribution in both samples was not in agreement (κ = −0.431). The ISH analysis revealed that HPV-DNA signal was observed in urothelial cells of five (55.7%) of nine detectable HPV-positive cytological samples. Six (66.7%) of nine HPV-positive cases had HPV-DNA signals in tumor tissue. The use of washing samples was likely applicable for investigating HPV prevalence in the urinary bladder. HPV-DNA detected in washing samples might be frequently derived from the urinary bladder.
1 INTRODUCTION
Human papillomavirus (HPV) infection, which is a causative agent for cervical cancer, can be transmitted between individuals through sexual contact, and numerous epidemiological studies demonstrated that the incidence of HPV infection in females and males is similar.1, 2 Therefore, HPV infection is considered one of the most common sexually transmitted infections.3-5 The common site of HPV infection in females and males is the external genitalia. The cervix and the vagina are the most common anatomic sites of HPV infection in females, whereas the glans, penis, coronal sulcus, and inner prepuce in males.1, 6 Contrastingly, the urinary tract has not been regarded as an important site of HPV infection in females and males.7
Urine is a most convenient and simple tool for screening HPV infection in the urinary tract; however, identifying the anatomic sites of HPV infection in the urinary tract in cases with HPV-deoxyribonucleic acid (DNA) detection in spontaneously voided urine is difficult. Indeed, epithelial cells exfoliated from the external genitalia, external orifice, and distal urethra are often contaminated in urine samples in females.8, 9 Contrastingly, HPV detection is the lowest in samples of the urogenital in males, such as the urethra, urine, and semen, and especially urine sample is reported to be inadequate for HPV detection.1, 6
However, we have recently reported HPV prevalence in urine samples by applying liquid-based cytology and demonstrated that the urinary tract can be an alternative frequent site of HPV infection in males.10, 11 One large epidemiological study used urine specimens that are collected from 845 outpatients in the urological clinic, detected HPV in 6.2% of urine samples, and revealed urethritis as an independent risk factor for HPV detection from urine samples.10 Additionally, Kawaguchi et al. mentioned that the HPV detection rate in urine specimens was 3.3% in healthy subjects and 21.0% in patients with urethritis, and demonstrated an HPV-DNA localization in urothelial cells by in situ hybridization (ISH).11 Therefore, we considered HPV infection to occur in the urinary bladder at a constant frequency.
Currently, HPV infection has been reported to be associated with the development of various cancers other than cervical cancer, and the relationship between HPV infection and oropharyngeal, anal, and penile cancers has been established.12-14 In recent years, the possibility that HPV infection may be involved in the development of bladder cancer has been discussed.7, 15, 16 HPV prevalence in cases of bladder carcinoma widely varies, and its etiological role in bladder cancer development remained inconclusive. However, HPV-DNA is likely certainly detected in some cases with bladder carcinoma.
The bladder washing sample is collected during cystoscopy, and washing can exfoliate large sheets of urothelium and even three-dimensional urothelial fragments.17 Bladder washing samples are a convenient tool for the cytological evaluation of the bladder mucosa and are likely suitable to investigate HPV prevalence in the urinary bladder. Additionally, cystoscopy is a routine examination to find and follow-up bladder tumor observation, and collecting washing samples is less invasive compared to bladder biopsy. Currently, only one report has examined HPV infection in washing samples among patients with bladder carcinoma.18
The present study aimed to elucidate the appropriateness of spontaneously voided urine or bladder washing in screening for HPV infection in the urinary bladder. Initially, we compared HPV detection and genotype distribution in urine and washing samples among the patients with bladder tumors receiving transurethral resection (TUR) and additionally confirmed HPV-DNA localization by ISH using cytological samples in cases with HPV-positive washing samples. Furthermore, we investigated the presence of HPV-DNA in bladder tumor tissues in these cases.
2 MATERIALS AND METHOD
2.1 Subjects and samples
A total of 201 patients with bladder tumors who underwent TUR between 2018 and 2020 at Kanazawa University Hospital (Kanazawa, Japan) were enrolled in the present analysis. Written informed consent for the use of all samples was obtained from all participants according to a protocol approved by the Ethics Committee of the Kanazawa University Graduate School of Medicine.
Initially, mid-streamed urine samples were obtained from each participant before operation. Next, bladder washing samples were also collected just before performing TUR. The resectoscope for TUR was inserted into the urinary bladder and then urine was completely removed. Then, approximately 100 ml of normal saline was instilled and recovered. This procedure was repeated once again, and consequently, this waste fluid was collected into a sterile beaker. Of both samples, 15 ml were placed into a separate tube. Each sample (15 ml) was centrifuged at approximately 500g for 5 min, and the sediment was placed into another tube containing 2.5 ml of preservative solution for liquid-based cytology (TACAS Amber; MBL Medical & Biological Laboratories Co., Ltd.) and stored at 4°C until use. After the operation, formalin-fixed, paraffin-embedded tumor tissue samples of all participants were also prepared. The diagnosis of a bladder tumor had been made by an experienced pathologist at our institution.
Demographic information, including patient age, gender, smoking rate, tumor location in the urinary bladder, pathological type, tumor grade, and invasiveness, was recorded for all participants.
2.1.1 HPV-DNA test and genotyping
Aliquots of 800 μl of both urine and washing samples were centrifuged at approximately 1500g for 5 min, and the supernatants were discarded. The cell pellets were washed twice with 300 μl of 10 mmol/L Tris-HCl (pH: 8.0). DNA was extracted from the cells using a DNA extraction kit (SMI Test; G&G Science Co.) following the manufacturer's instructions.
DNA quality was initially confirmed by amplifying the beta-globin gene as an internal control by polymerase chain reaction (PCR), which revealed positive results in all samples.19 HPV-DNA was examined by a nested PCR with GP5+/6+ and MY09/11 primers as previously described.20 HPV genotyping was performed by flow-through hybridization using an HPV GenoArray Diagnostic Kit (Hybribio Ltd.).19 This assay can determine 21 HPV genotypes, including 15 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) and 6 low-risk HPV types (6, 11, 42, 43, 44, and 81). Currently, more than 200 types of HPV have been isolated and at least 40 types of mucosal HPV types have been identified. Therefore, some samples, with positive nested PCR but negative 21 genotyping test results,21-23 were defined as representing the unknown (UK) HPV genotypes.
2.1.2 ISH of washing cytological samples
ISH was performed to confirm the presence of HPV-DNA in urothelial cells contained in the HPV-positive washing samples as described previously.20 Washing samples of 700 μl preserved in liquid-based cytology solution were centrifuged at 500g for 10 min. The supernatant was discarded, and cell pellets were resuspended in phosphate-buffered saline to repeat the washing step. After three rounds of washing, cell pellets were fixed in 4% paraformaldehyde (PFA) and allowed to air dry on glass microscope slides.20
All ISH procedures were performed using a commercial HPV detection kit following the manufacturer's instructions (Dako GenoPoint System K0620; Dako). Denatured DNA was hybridized with a wide-spectrum biotinylated HPV-DNA probe (Y1404; Dako) to recognize 15 HPV genotypes (6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). HPV-DNA signals were visualized as brown staining by a diaminobenzidine reaction, and the slides were counterstained with hematoxylin.11 ISH was also performed in five HPV-negative samples as an experimental control.
2.1.3 ISH of bladder tumor tissues
We tried to demonstrate HPV-DNA localization in bladder tumor samples in HPV-positive washing samples, and ISH analysis was performed using paraffin-embedded tumor tissue samples obtained by TUR-Bt following previous-described sample protocol.24
2.2 Statistical analysis
The κ test was used to analyze the agreement of HPV types in urines and washing samples. The κ coefficient takes values from −1 to 1, and the degree of agreement is determined as follows: <0 indicated no agreement, 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement. The Mann–Whitney U test was used to compare age distribution between the HPV-positive and negative groups in the bladder washing samples. The χ2 test was used to compare the characteristics of the two groups. In all analyses, results were considered statistically significant with p < 0.05. Statistical analyses were performed using Statistical Package for the Social Sciences version 25 (IBM Corp.).
3 RESULTS
3.1 Demographic and clinicopathologic information
Demographic and clinicopathological information of a total of 201 participants is shown in Table 1. Their ages ranged from 51 to 93 years (mean age: 74.4 years), with 167 males (83.0%) and 34 females (17.0%). Smoking was reported in 140 patients (69.7%). The most common pathological type was urothelial carcinoma (UC) in 188 (93.5%) patients, followed by UC/squamous cell carcinoma in 7 (3.5%), UC/adenocarcinoma in 3 (1.5%), and benign bladder papilloma in 3 (1.5%). Histologically, out of 198 patients with bladder carcinoma excluding papilloma, 49 (24.7%) had low-grade tumors and 149 (75.3%) had high-grade tumors. Noninvasive tumors were determined in 131 patients (66.2%), invasive cancer in 61 (30.8%), and unknown invasiveness in 6 (3.0%). There were no patients affected by additional diseases such as HPV-driven tumors of the upper respiratory tract, anal cancer, penile cancer, and cervical neoplasms.
| Variables | |
|---|---|
| Age mean (range) years | 74.4 (51–93) |
| Gender | |
| Men (%) | 167 (83.0) |
| Women (%) | 34 (17.0) |
| Smoking (%) | 140 (69.7) |
| Tumor Type | |
| UC (%) | 188 (93.5) |
| UC + SCC (%) | 7 (3.5) |
| UC + adenocarcinoma (%) | 3 (1.5) |
| Bladder papilloma (%) | 3 (1.5) |
| Tumor grade (n = 198) | |
| Low grade (%) | 49 (24.7) |
| High grade (%) | 149 (75.3) |
| Pathologic invasion (n = 198) | |
| Noninvasive (Ta) (%) | 131 (66.2) |
| Invasive (T1 or more) (%) | 61 (30.8) |
| Unknown (%) | 6 (3.0) |
- Abbreviations: SCC, squamous cell carcinoma; UC, urothelial carcinoma.
3.1.1 HPV prevalence and type distribution in urine and washing samples
In all urine and bladder washing samples, a beta-globin gene could be amplified. HPV-DNA was detected in 19 (9.5%) urine samples and 14 (7.0%) washing samples (Table 2). High-risk HPV prevalence in urine and washing samples was 7.5% and 4.0%, respectively. Regarding HPV type distribution, the most common HPV type was HPV 16, followed by HPV 52 in both samples.
| Urine, n (%) | Bladder washings, n (%) | |
|---|---|---|
| HPV prevalence | ||
| Any risk types | 19 (9.5) | 14 (7.0) |
| High-risk type | 15 (7.5) | 8 (4.0) |
| Low-risk type | 10 (5.0) | 4 (2.0) |
| Unknown type | 1 (0.5) | 4 (2.0) |
| High risk | ||
| HPV 16 | 6 (31.6) | 4 (28.5) |
| HPV 18 | 2 (10.5) | 1 (7.1) |
| HPV 39 | 1 (5.3) | 0 |
| HPV 51 | 1 (5.3) | 0 |
| HPV 52 | 3 (15.8) | 2 (14.3) |
| HPV 58 | 1 (5.3) | 0 |
| HPV 59 | 1 (5.3) | 0 |
| HPV 68 | 0 | 1 (7.1) |
| Low risk | ||
| HPV 11 | 3 (15.8) | 2 (14.3) |
| HPV 42 | 3 (15.8) | 0 |
| HPV 43 | 1 (5.3) | 0 |
| HPV 44 | 2 (10.5) | 2 (14.3) |
| HPV 81 | 1 (5.3) | 0 |
| Unknown | 1 (5.3) | 4 (28.5) |
- Abbreviation: HPV, human papillomavirus.
3.1.2 Comparisons in HPV positivity and genotype in both samples
HPV was detected in both urine and washing samples in five cases. Two cases have partially matched HPV types and no cases with completely matched HPV types (κ coefficient = −0.403) (Table 3). Therefore, the use of bladder washing samples was likely to be applicable for investigating HPV prevalence in the urinary bladder. Then, ISH analysis in cytology and bladder tumor tissue was performed only in HPV-positive bladder washing samples.
| Urine/Washing | κ | |||||||
|---|---|---|---|---|---|---|---|---|
| +/+ | +/− | −/+ | −/− | |||||
| HPV positive | 5 | 14 | 9 | 168 | −0.403 | |||
| Detail of HPV-positive cases in both samples | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case | Tumor type | HPV types | ||||||
| Urine | Washing | |||||||
| 2 | Invasive UC | HPV 16, 52 | HPV 52 | |||||
| 3 | Papilloma | HPV 16, 18 | HPV 16, 44 | |||||
| 7 | Noninvasive UC | HPV 18 | HPV 11 | |||||
| 10 | Invasive UC | HPV 42 | HPV 44 | |||||
| 14 | Invasive UC | HPV 43, 59 | HPV UK | |||||
- Abbreviations: HPV, human papillomavirus; UC, urothelial carcinoma; UK, unknown type.
3.1.3 Comparisons in patients' characteristics between HPV-positive and negative washing samples
Among the washing samples, no significant difference was found in age, gender, smoking status, cytological findings, tumor type, tumor grade, and tumor invasion (Table 4). HPV-DNA was detected in all cases of inverted papilloma, although without significant differences.
| Variables | HPV-positive (n = 14) | HPV-negative (n = 187) | p |
|---|---|---|---|
| N (%) | N (%) | ||
| Age mean (range) years | 71.9 (51–92) | 74.6 (52–93) | 0.358 |
| Gender | 0.640 | ||
| Men | 11 (78.6) | 156 (83.4) | |
| Woman | 3 (21.4) | 31 (16.6) | |
| Smoking | 0.450 | ||
| Smoking | 8 (57.1) | 132 (70.6) | |
| Nonsmoking | 6 (42.9) | 55 (29.4) | |
| Tumor localization | 0.843 | ||
| Single | 9 (64.3) | 108 (57.8) | |
| Multiple | 5 (35.7) | 79 (42.2) | |
| Cytological findings | 0.382 | ||
| Positive | 6 (42.9) | 62 (33.2) | |
| Suspicious | 0 | 34 (18.2) | |
| Negative | 8 (57.1) | 91 (40.6) | |
| Tumor type | 0.588 | ||
| UC | 10 (57.1) | 178 (78.6) | |
| UC + SCC | 0 | 7 (1.0) | |
| Adenocarcinoma | 1 (7.1) | 2 (0.5) | |
| Bladder papilloma | 3 (14.3) | 0 | |
| Tumor grade (n = 198) | 0.873 | ||
| Low grade | 3 (27.3) | 46 (24.6) | |
| High grade | 8 (72.7) | 141 (75.4) | |
| Pathologic invasion (n = 198) | 0.074 | ||
| Noninvasive (Ta) | 3 (27.3) | 128 (68.4) | |
| Invasive (T1 or more) | 7 (63.6) | 54 (28.9) | |
| Unknown | 1 (9.1) | 5 (2.7) |
- Abbreviations: HPV, human papillomavirus; UC, urothelial carcinoma; SCC, squamous cell carcinoma.
3.1.4 ISH analysis in cytological samples and tumor tissues
The HPV-DNA probe used in the present study was detectable for 2 low-risk and 13 high-risk HPV genotypes. Therefore, the ISH analysis results were available in the washing samples and tumor tissue in nine cases (detectable HPV-positive cases) where the prior PCR result was the genotypes, which were detectable by the ISH HPV-DNA probe (Table 5). Among the washing samples, an HPV-DNA signal was observed in the nuclei of urothelial cells in five detectable HPV-positive samples (5/9 cases; 55.7%). However, HPV-DNA signals were observed in many atypical urothelial cells in only one case (Figure 1A), whereas the other case had HPV-DNA signals in sporadic urothelial cells (Figure 1B). Contrastingly, six (66.7%) of nine detectable HPV-positive cases had HPV-DNA signals in the nuclei of tumor cells (Figure 2A). HPV-DNA signal was confirmed in tumorous tissues among all bladder papilloma cases (Figure 2B). Thus, eight cases were positive for ISH analysis in either cytological or tumor tissue samples.
| Case | HPV types | Age | Gender | Tumor localization | Cytology | Histopathology | ISH analysis | |
|---|---|---|---|---|---|---|---|---|
| Washing | Tissue | |||||||
| Detectable HPV-positive casesa | ||||||||
| 1 | 16 52 | 71 | M | Single | - | Papilloma | + | + |
| 2 | 52 | 81 | M | Single | UC | UC, high grade, invasive | - | + |
| 3 | 16 44 | 51 | M | Single | - | Papilloma | - | + |
| 4 | 16 | 76 | M | Multiple | UC | UC, high grade, invasive | + | - |
| 5 | 11 | 66 | F | Single | - | Adenocarcinoma | - | + |
| 6 | 68 | 60 | F | Single | - | UC, low grade, noninvasive | - | - |
| 7 | 11 | 70 | M | Multiple | - | UC, low grade, noninvasive | + | - |
| 8 | 16 | 53 | M | Multiple | - | Papilloma | + | + |
| 9 | 18 | 92 | M | Multiple | UC | UC, high grade, noninvasive | + | + |
| Nondetectable HPV-positive cases | ||||||||
| 10 | 44 | 69 | F | Multiple | UC | UC, high grade, invasive | - | - |
| 11 | UK | 69 | M | Single | - | UC, high grade, invasive | - | - |
| 12 | UK | 78 | M | Single | - | UC, low grade, noninvasive | - | - |
| 13 | UK | 82 | M | Single | UC | UC, high grade, invasive | - | - |
| 14 | UK | 89 | M | Single | UC | UC, high grade, invasive | - | - |
- Abbreviations: ISH, in situ hybridization; HPV, human papillomavirus; M, male; F, female; UC, urothelial carcinoma; UK, unknown type.
- a The cases where the prior PCR result was the genotypes that were detectable by ISH HPV-DNA probe (type 6, 11, 16,18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68).
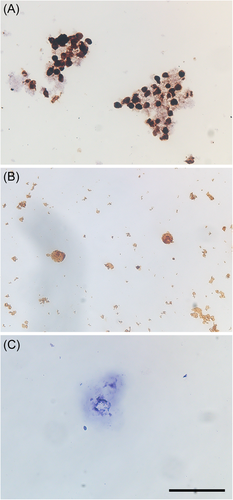
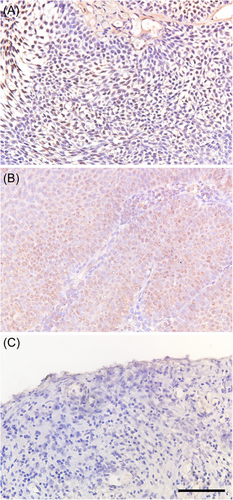
Moreover, in all 5 cases (4 UK type and 1 type 44) with HPV-genotype detection, which was not matched with ISH HPV-DNA probe (non-detectable HPV-positive cases), and 5 HPV-negative cases, HPV-DNA signals were not observed in the washing samples and tumor tissue (Table 5 and Figures 1C and 2C). Generally, ISH has problems with low specificity; however, the accuracy of ISH analysis conducted in the present study was fully acceptable.
4 DISCUSSION
Several previous studies reported HPV prevalence in urine samples in males10, 11; however, the origin of HPV infection in anatomic sites of the urinary tract could not be determined. In the present study, HPV-DNA prevalence was 9.5% in the urine and 7.0% in the washing samples and was slightly higher in urine compared to washing samples. Contrastingly, HPV type distribution in both samples was not in agreement (κ = −0.431), suggesting that HPV detection in urine does not necessarily reflect HPV infection in the urinary bladder. HPV detection in urine samples may be due to the contamination of HPV-infected cells from the external urethral orifice or distal urethra, which are the most common anatomic sites of HPV infection among the urinary tract in females and males.1, 6, 8, 9 Therefore, urine samples were unlikely to be suitable for HPV infection detection in the urinary bladder.
The present study collected all the subjects from patients with bladder tumors. A recent meta-analysis to investigate the relationship between HPV infection and bladder cancer described a relatively high HPV prevalence (14.3% [95% confidence interval [CI]: 8.9%–22.2%]), although without significant association (odds ratio [OR]: 2.077, 95% CI: 0.940%–4.587%).16 Furthermore, one large epidemiological study that used urine specimens collected from 845 outpatients in the urological clinic more frequently detected HPV-DNA in patients with urogenital tumors, when analyzing excluding urethritis cases.10 Therefore, we considered that a sufficient number of HPV-positive cases in both samples could be obtained in patients with bladder tumors to successfully compare HPV prevalence in urine and bladder washings samples.
Only one previous study reported HPV prevalence in frozen bladder washing samples.18 This report demonstrated that HPV-DNA and high-risk HPV-DNA was 15.2% and 8.1%, respectively, in washing samples of 166 bladder carcinoma patients, and high-risk HPV prevalence significantly increased with tumor grade and stage progressions.18 Contrastingly, HPV prevalence (7.0%) found in our study was far less compared to one reported by Moonen, which was not correlated with any patients' background, including tumor grade and stage, probably due to differences in patients' characteristics and race. Studies that examined HPV prevalence in bladder washing samples have been extremely limited; thus, further studies including a large number of subjects are needed.
In cases with HPV-positive washing samples, ISH analysis was conducted to confirm HPV-DNA localization in urothelial cells, which is likely to be unique. To our knowledge, this is the first report to demonstrate HPV-DNA signals in urothelial cells using cytological samples. Among the nine detectable HPV-positive cases, HPV-DNA signals could be observed in five, suggesting that HPV detected in washing samples was derived from urothelial cells of the bladder epithelium. However, HPV-DNA signals could be observed only in slight sporadic urothelial cells in many cases. The fixed cells were gradually removed via ISH, and consequently, the number of cells that could be analyzed decreased. Therefore, a negative result in four cases with inadequate HPV-DNA signals may not be always caused by urethral contamination but may be due to the low sensitivity of ISH analysis in cytological samples. Further improvement in ISH methods is likely to be required.
ISH analysis was also performed in bladder tumor samples in all HPV-positive washing samples in the present study. Of nine detectable HPV-positive cases, six (66.7%) had HPV-DNA signals in the nuclei of tumor cells, suggesting that HPV-DNA detected in washing samples might be derived from the bladder tumor tissue. Contrastingly, in two cases with positive HPV-DNA signals in cytological samples and negative signals in tumor tissues, HPV infection may have occurred in the normal bladder mucosa, but not in the bladder tumor. In one case (female, HPV 68-positive case) without evidence of HPV-DNA signals in both cytological and pathological ISH analysis, the result may be due to a false-negative ISH or HPV-infected cells urethral contamination. However, the number of cases that could have been evaluated by ISH (only nine detectable HPV-positive cases) was limited; thus, further studies including many cases are required to establish more conclusive findings.
The present study demonstrated that HPV-DNA signals could be observed in tumor tissues in six cases. However, the present study cannot mention a correlation between HPV infection and bladder carcinoma, which is an interesting topic to be debated. The relationship between bladder cancer and HPV remained debatable, and some reports suggested a potential correlation between bladder cancer and HPV infection.15, 24-26 A most recent meta-analysis that analyzed 52 studies comprising 2855 cases showed an incidence between 0% and 100% for HPV in bladder cancer and concluded that the role of HPV in bladder carcinoma remained nonconsensual.16 Interestingly, HPV 16 was detected in three bladder papilloma cases, which is a relatively rare benign bladder tumor. Two previous studies suggested an etiological role of HPV infections in bladder papilloma development, with its prevalence rate of 60%–87%.27, 28 HPV 16 was the common genotype in bladder papilloma, consistent with the present findings.27 Contrastingly, some reports denied the relationship between HPV infection and papilloma29, 30 and the role of HPV infection in the etiology of bladder papilloma has also provided conflicting evidence.
We demonstrated that HPV infection could occur in the urinary bladder. Many previous studies on HPV infection in the urinary bladder have subjects that were limited to bladder tumor tissues obtained from patients with bladder cancer due to its higher invasiveness of sampling under anesthesia.7, 24, 26-28, 30-33 Therefore, few studies on HPV infection in the urinary bladder among the cases without bladder tumors have been currently available.33 Additionally, the natural history of HPV infection in the urinary bladder remained unknown. Therefore, clarifying the persistence of urinary HPV infection in the urinary bladder for a long period is necessary to elucidate the pathogenicity of HPV infection in the urinary tract. Washing samples can be collected less invasively compared to bladder mucosa biopsy, and are available for the examination of HPV infections in the urinary bladder. By investigating HPV infection in bladder washing samples collected continuously at follow-up cystoscopic observation after operation among HPV-positive cases, natural history of HPV infection in urinary bladder can be elucidated in the future.
The present study had some limitations. First, the total number of subjects is low, and consequently, only 14 HPV-positive cases were included. Particularly, the number of detectable HPV-positive cases for ISH analysis was extremely limited (n = 9). Therefore, this is likely inadequate to calculate the sensitivity of washing samples to bladder HPV infection. Further studies, including a large population, are required to reach a more definite conclusion. Second, healthy control subjects were not included. However, the present study was not conducted to compare HPV prevalence between the cases with a bladder tumor and healthy controls but to evaluate the appropriateness of bladder washing samples for investigating HPV infection in the urinary bladder by targeting the subjects with bladder cancer. Since our present study was conducted to investigate whether bladder washing samples was likely applicable for investigating HPV prevalence in the urinary bladder, healthy control was not included because of higher invasive samplings. However, we are planning a large-scale study including healthy subjects for further understanding HPV infection in the urinary bladder. Additionally, we cannot completely deny the possibility that contaminations of HPV-infected cells from other urinary tracts were included in washing samples. Indeed, in the present study, one case had no evidence of HPV-DNA signals in both cytological and pathological ISH analysis in HPV-positive washing samples. Furthermore, collecting washing samples is accompanied by some invasiveness with discomfort and pain, although it is less invasive compared to bladder biopsy. Washing samples can be obtained only by catheterization or cystoscopic examination, which certainly interferes with a test for healthy people.
5 CONCLUSIONS
HPV prevalence and genotype detected in the urine and bladder washing samples were not consistent, suggesting that the use of bladder washing samples was likely applicable for investigating HPV prevalence in the urinary bladder. The present study demonstrated that HPV-DNA detected in washing samples might be frequently derived from the urinary bladder. The natural history of bladder HPV infection can be examined using washing samples, which is an important issue for understanding the pathogenesis of HPV infection in the urinary tract.
AUTHOR CONTRIBUTIONS
Tomomi Nakagawa: Study concept and design, data collection, statistical analyses and data interpretation, and drafting of the manuscript. Kazuyoshi Shigehara: Study concept and design, data collection, statistical analyses and data interpretation, critical revision of the manuscript and final approval of the version to be published. Yuki Kato: Data collection. Shohei Kawaguchi: Study concept and design, data collection, statistical analysis, and data interpretation. Hiroki Nakata: Statistical analyses and data interpretation. Taito Nakano: Data collection. Kouji Izumi, Yoshifumi Kadono, and Atsushi Mizokami Final approval of the version to be published.
ACKNOWLEDGMENTS
The authors wish to thank Enago (www.enago.jp) for the English language review. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This investigation was approved by the Ethics Committee of Kanazawa University Graduate School of Medicine (Approval No. 2017-075).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




