Broadly neutralizing antibodies recognizing different antigenic epitopes act synergistically against the influenza B virus
Linlin Zhai, Limin Zhang, Yushan Jiang, Baisheng Li, Minghui Yang, Wei Zhao, Yixin Chen, and Chenguang Shen contributed equally to this study.
Abstract
The discovery of broadly neutralizing monoclonal antibodies against influenza viruses has raised hope for the successful development of new antiviral drugs. However, due to the speed and variety of mutations in influenza viruses, single-component antibodies that recognize specific epitopes are susceptible to viral escape and have limited efficacy when administration is delayed. Hence, it is necessary to develop alternative strategies with better antiviral activity. Influenza B virus infection can cause severe illness in children and the elderly. Commonly used anti-influenza drugs have low clinical efficacy against influenza B virus. In this study, we investigated the antiviral efficacy of combinations of representative monoclonal antibodies targeting different antigenic epitopes against the influenza B virus. We found that combinations of antibodies recognizing the hemagglutinin (HA) head and stem regions showed a stronger neutralizing activity than single antibodies and other antibody combinations in vitro. In addition, we found that pair-wise combinations of antibodies recognizing the HA head region, HA stem region, and neuraminidase enzyme-activated region showed superior antiviral activity than single antibodies in both mouse and ferret in vivo protection assays. Notably, these antibody combinations still displayed good antiviral efficacy when treatment was delayed. Mechanistic studies further revealed that combining antibodies recognizing different epitope regions resulted in extremely strong antibody-dependent cell-mediated cytotoxicity, which may partly explain their superior antiviral effects. Together, the findings of this study provide new avenues for the development of better antiviral drugs and vaccines against influenza viruses.
1 INTRODUCTION
Infections caused by the influenza A and B viruses are a persistent public health threat, with annual influenza epidemics generally infecting 5%−15% of the global population and resulting in 3−5 million severe cases and 290 000−650 000 deaths.1-4 The widespread impact of influenza viruses is mainly attributed to the scarcity of effective antiviral drugs and vaccines.5
Influenza viruses contain two major surface glycoproteins, hemagglutinin (HA), and neuraminidase (NA). The HA protein is the most abundant membrane protein on the surface of influenza viruses: the HA head region is responsible for receptor binding, while the HA stem region can mediate viral membrane fusion with cellular membranes to release the viral genome. Consequently, the HA head and stem regions are both important targets for broadly neutralizing antibodies against the influenza virus.6 NA, the second major surface protein on influenza viruses, cleaves the terminal sialic acid residue from N-linked glycans to facilitate virus excretion from infected cells and the release of viruses captured by natural defense proteins, such as mucins.7, 8 Therefore, antibodies targeting NA block influenza virus replication by interfering with the release of viral progeny.9 Since the receptor binding sites for the HA head region, HA stem region, and NA protease active center are highly conserved, they have been the main targets for the development of antiviral drugs and vaccines against influenza.10, 11
In recent years, multiple broadly neutralizing monoclonal antibodies targeting the HA head region, HA stem region, or NA have been reported to neutralize influenza viruses from different evolutionary branches and display better antiviral efficacy than oseltamivir, raising hope for the development of promising novel antiviral drugs and vaccines.12, 13 Although broadly neutralizing antibodies recognizing specific epitopes are able to neutralize influenza viruses with different evolutionary relationships, some mutations can still allow the virus to escape neutralization by these antibodies, thus limiting their clinical applications.14 Several studies related to broad-spectrum monoclonal antibodies against novel coronaviruses and human immunodeficiency viruses have suggested that a cocktail of antibodies recognizing different epitopes can exert superior antiviral effects with a broader spectrum of neutralization against different viral strains.15, 16 Thus, cocktails of multiple broadly neutralizing monoclonal antibodies recognizing different influenza epitopes could display better antiviral activity and antiviral escape activity, and be more promising in terms of their clinical applications. Unfortunately, no studies have yet been conducted to investigate the synergistic antiviral effects of broadly neutralizing antibodies that recognize different influenza virus epitopes.
In previous studies, our research group has obtained a panel of influenza B virus strains from various lineages, generated or expressed a batch of broadly neutralizing antibodies against influenza B virus, and established a comprehensive evaluation platform for influenza B virus antibody which consists of reasonable in vitro and in vivo assays. Therefore, in this study, we characterized the in vitro and in vivo synergistic antiviral activity of cocktails of three representative classes of broadly neutralizing monoclonal antibodies against the influenza B virus. 12G6 antibodies recognize the HA head region receptor binding site and cross-neutralize influenza B viruses that have evolved since 1940, including those from the Yamagata, Victorian, and early lineages.17 5A7 antibodies recognize the HA stem region and broadly neutralize influenza B virus strains belonging to the Yamagata and Victorian lineages isolated from 1985 to 2006.18 2E01 antibodies recognize NA and exert broad protective effects in vivo by blocking the NA active site using the long CDR-H3 loop to inhibit NA activity, similar to NA inhibitors such as oseltamivir.11 Since these three types of antibodies recognize different representative targets on the influenza virus, the antibody cocktails may have synergistic antiviral effects19 and could therefore be used to develop high-efficacy therapeutic agents and vaccines against the influenza virus.
2 METHODS AND MATERIALS
2.1 Study design
The objective of this study was to evaluate the antiviral effects of single 12G6, 5A7, and 2E01 antibodies and 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 antibody combinations against the influenza B virus in vitro and in vivo. All in vivo studies were performed according to the guidelines of the Institutional Animal Care and Use Committee and were approved by the Ethics Committee of the Laboratory Animal Center. Six-week-old female BALB/c mice were purchased from Shanghai Silaike Laboratory Animal Co., Ltd. Ferrets were purchased from Wuxi Sangosho Biotechnology Co., Ltd. All animals were maintained in individual ventilated cages and were monitored closely for survival and signs of illness for up to 14 days after viral challenge. All in vivo experiments followed strict guidelines for humane endpoints; animals that lost over 25% of their initial body weight were immediately euthanized by CO2 asphyxiation and were recorded as non-surviving. All animals were randomly assigned to treatment groups using the randomization tool in Microsoft Excel. All in vitro and in vivo experiments were repeated at least three times unless otherwise indicated in the figure legends.
2.2 Virus and cells
B/Harbin/7/1994, B/Florida/4/2006, B/Massachusetts/02/2012, B/Hong Kong/330/2001, B/Malaysia/2506/2004, and B/Brisbane/60/2008 influenza virus strains were provided by BEI Resources. Other influenza B strains were collected from the First Hospital of Xiamen University and Xiamen International Travel Healthcare Center. Mouse-adapted (MA)-B/Florida/4/2006 and MA-B/Hong Kong/330/2001 strains were generated in our laboratory through successive passages in the lungs of mice. All viruses were cultured in MDCK cells in Dulbecco Modified Eagle's Medium (DMEM) containing 10% calf serum using standard viral culture techniques. To concentrate and purify the viruses, the infected cell supernatant was subjected to high-speed centrifugation through a 30% sucrose buffer. Purified viruses were resuspended in phosphate-buffered saline (PBS) and stored at −80°C until use. The antibodies used in this study were expressed in 293T eukaryotic cells cultured in DMEM containing 10% calf serum and purified using Protein A media affinity chromatography columns.
2.3 Antibody expression and purification assay
Heavy chain plasmids and light chain plasmids containing the 12G6, 5A7, and 2E01 genes were transfected and expressed in 293T cells in a 1:1 ratio 1 day before the experiment to ensure that the cells reached 70%−80% density before transfection, depending on the cell growth rate. Serum-free medium was added to two clean sterile eppendorf) tubes and along with plasmids and transfection reagent PEI (Proteintech), and incubated at 37°C for 5 min. PEI was then slowly added to the DNA, gently mixed, and incubated at 37°C for 15 min. The PEI and plasmid complexes were added to the cells (according to the manufacturer's instructions) and gently shaken to ensure an even distribution. 293T cell supernatant was collected and purified using Protein A chromatography columns. Briefly, the cell supernatant was centrifuged at 20 000 rpm for 30 min and filtered through a 0.22 µm pore size membrane using an extraction pump. The column was first loaded with 10 ml of Sepharose 4B medium coupled with Protein A and then connected to an AKTA Explorer 100 system. Pump A was connected to 0.2 M disodium hydrogen phosphate solution and pump B was connected to 0.2 M citric acid solution. The detection wavelength was set to UV 280 nm, with a flow rate of 5 ml/min. To adjust the A/B pump injection ratio, the column was first rinsed with 100% B solution (pH 2.3) to remove protein impurities and replaced with 10% B solution (pH 8.0) to equilibrate the pH. Once the detection wavelength had stabilized to zero, the sample was loaded. After the penetration peak had passed, 10% B solution was added to continue equilibration until the detection wavelength had stabilized to zero. The elution peak was detected using 70% B solution (pH 4.0). Crude and pure antibodies were washed and concentrated in a 30 kDa concentration tube, centrifuged at 3500×g for 30−60 min, and washed three times with PBS to obtain purified antibodies.
2.4 Enzyme-linked immunosorbent assay (ELISA)
Microtiter plates (WANTAI BioPharm) were coated with 5 μg/ml purified viruses (50 μl per well) overnight at 4°C, washed three times with PBS containing 0.1% v/v Tween-20 (PBST), blocked in PBS containing 2% w/v nonfat dry milk (blocking solution) for 2 h at 37°C, and then washed with PBST. Serial twofold dilutions of the purified antibodies were added to the wells and incubated at 37°C for 30 min. After three washes, 100 μl of horseradish peroxidase-conjugated goat anti-mouse (or anti-human) IgG antibody solution was added to each well and incubated at 37°C for 30 min. After five washes, 100 μl of tetramethylbenzidine substrate (WANTAI BioPharm) was added to the plate at room temperature in the dark and the reaction was stopped after 15 min using 2 M H2SO4 solution. The absorbance was measured at 450 nm. All samples were run in triplicate. The relative affinity of antibody binding to purified viruses was determined by measuring the concentration of each antibody required to achieve the EC50. Competitive ELISA was performed as described previously,17 with an extra preincubation step for unlabeled excess competitive antibodies before the addition of antibodies to the microtiter wells. Competition levels were calculated as the percentage inhibition of half the maximum binding concentration of the tested antibody, relative to the absorbance value without the addition of the competitor.
2.5 Microneutralization assay
Microneutralization assays were carried out as described previously.20 Briefly, MDCK cells were maintained in DMEM supplemented with 10% calf serum at 37°C with 5% CO2. On the day of the experiment, MDCK cells in a 96-well plate were washed twice with PBS and incubated in DMEM supplemented with 3 μg/ml trypsin-EDTA. Serial twofold dilutions of each antibody were mixed with an equal volume of virus and incubated for 2 h at 37°C. After incubation, 35 µl of the mixture containing 100 times the TCID50 (50% tissue culture infectious dose) of the virus was added to the MDCK cells and allowed to adsorb for 1 h. The viral supernatant was removed and replaced with DMEM containing antibiotics. Cells were cultured for 72 h at 37°C with 5% CO2 and the neutralization titer was determined using the HA test in quadruplicate. Briefly, 50 µl of 0.5% TRBCs was added to 50 µl of cell culture supernatant and incubated at room temperature for 1 h. The neutralization titer was defined as the lowest antibody concentration that was negative for hemagglutination.
2.6 Prophylactic and therapeutic efficacy studies in mice
For the prophylactic experiments, 6−8-week-old female BALB/c mice were injected intravenously with 200 µl of a control antibody, single 12G6, 5A7, or 2E01 antibodies, or 12G6 + 5A7, 12G6 + 2E01, or 5A7 + 2E01 combination antibodies (20 mg/kg; n = 6 per group). After 1 day, mice were anesthetized using isoflurane and oxygen, and infected intranasally with standard viral cultures (25 MLD50) of MA-B/Florida/4/2006 (Yamagata) or MA-B/Hong Kong/330/2001 (Victoria) grown in MDCK cells. After 1, 3, or 5 days, the mice were injected intravenously with 200 µl of control IgG, single 12G6, 5A7, or 2E01 antibodies, or 12G6 + 5A7, 12G6 + 2E01, or 5A7 + 2E01 combination antibodies (10 mg/kg). The lungs of each mouse were collected 4 days after infection for viral titration. All animals were observed daily for mortality and morbidity, and body weight was measured 14 days postinfection.
2.7 Therapeutic efficacy studies in ferrets
The 14-week-old male ferrets used in this study were certified as being free of any evidence of infectious, contagious, or transmissible diseases by the vendor. All ferrets were transferred to a biosafety level 2 laboratory for at least 14 days of domestication before the experiment. Baseline weights and temperatures were measured at least 7 days before the experiment. A programmable temperature transponder (IPTT-300; Biomedical Data System) was implanted subcutaneously between the scapulae to measure subcutaneous body temperature twice daily. For the experiments, the ferrets and were divided into seven groups (n = 4), anesthetized using isoflurane, and injected with 500 µl of the viral reservoir prepared from infected MDCK cells per nostril (a total of 1 ml containing 1 × 107 TCID50). The ferrets were then held upright with their heads tilted backward slightly for approximately 1 min to reduce the likelihood of the inoculum dripping from the nostrils and were returned to their cages. After 24 h, the ferrets were injected intravenously with control antibodies, single 12G6, 5A7, or 2E01 antibodies, or 12G6 + 5A7, 12G6 + 2E01, or 5A7 + 2E01 combination antibodies (10 mg/kg). Body temperature was measured twice daily (8:00 a.m. and 8:00 p.m.) and nasal washes were collected daily.
2.8 Antibody-dependent cell-mediated cytotoxicity (ADCC) assay
ADCC was determined using a previously described flow cytometry-based assay employing two fluorescent dyes to distinguish between viable and dead cells,21 with slight modifications. Briefly, MDCK cells infected with influenza B viruses at an multiplicity of infection of 10 were labeled with PKH67 (Sigma-Aldrich), a general cell membrane-labeling dye. The cells were then washed twice with PBS, added to 96-well plates (5 × 104 cells per well) in triplicate, and incubated with serial dilutions of single antibodies or antibody combinations (0.5−20 mg/µl) for 30 min at 37°C. NK cells (natural killer cells) isolated from mouse spleens were added to the plates (1 × 106 per well), incubated at 37°C for 3 h, and treated with 1 µl of 7-AAD (eBioscience) to stain dead cells. Cell death was determined using a FACSAria III flow cytometer with BD FACS Diva software (BD Biosciences). The relative proportions of four identifiable cell populations in 10 000 target cells were determined through software analysis: live effector cells (no dye), dead effector cells (7-AAD positive), live target cells (PKH-67 positive), and dead target cells (PKH-67 and 7-AAD double positive). The assay controls used to determine the cell populations included target cells alone (background cell death) and target cells with 5 µl of Triton X-100 (maximum fluorescence). ADCC was expressed as a percentage as follows: ([percentage experimental lysis−percentage spontaneous lysis]/[percentage maximum lysis−percentage spontaneous lysis]) × 100. Percentage spontaneous lysis refers to the percentage lysis of infected cells with effector cells in the absence of antibodies. Percentage maximum lysis refers to the percentage lysis of infected cells with effector cells in the presence of 1% Triton X-100. Three ADCC assays were performed with NK cells from three different mice. Representative data are shown.
2.9 Statistical analysis
Data were presented as the mean ± SEM of three repeated experiments. To identify significant differences between survival curves, we used the log-rank test in GraphPad Prism 6.0. Significant differences in body weight were analyzed using the area under the curve (AUC), as described previously.17 Weight change was determined relative to the baseline weight of each mouse measured on Day 0. The AUC was defined as the summation of the area above and below the baseline. Mean AUC values were compared using analysis of variance with Dunnett's T3 adjustment for multiple comparisons. Statistical analyses were performed using SPSS software. Viral titration data for infected mice and the ADCC and CDC experiments were analyzed using multiple t-tests in GraphPad Prism 6.0. p values reported in the figures and figure legends indicate the following significance levels: *p < 0.05, **p < 0.01, and ***p < 0.001.
3 RESULTS
3.1 12G6, 5A7, and 2E01 recognize influenza B virus without competition
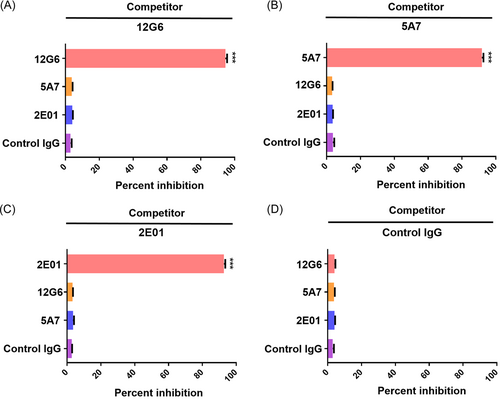
Antibodies that recognize different antigenic epitopes can exert synergistic antiviral effects.22 Previous studies have reported that the 12G6 antibody recognizes an epitope located in the HA head region of influenza B virus, with some overlap with the viral receptor binding region and >98% amino acid conservation at the recognized epitope.17 Meanwhile, the 5A7 antibody has been reported to recognize an epitope in the HA stem region of influenza B virus18 and the 2E01 antibody is thought to recognize conserved residues in the active site of influenza B virus NA.11 To verify that 12G6, 5A7, and 2E01 antibodies bind to influenza B virus without competition, do not compete for antigen recognition, ELISA competition assays were performed. The results of this assay verified that the three antibodies 12G6, 5A7, and 2E01 did not compete with any other antibodies except themselves (Figure 1).
3.2 Antibodies targeting different epitopes exert synergistic effects against influenza B virus in vitro
To determine whether antibodies targeting different epitopes exerted synergistic effects against influenza B virus in vitro, we first tested the binding activity of single 12G6, 5A7, and 2E01 antibodies and 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 antibody combinations against four Yamagata lineage viruses isolated during different years (B/Harbin/7/1994, B/Florida/4/2006, B/Massachusetts/02/2012, B/Xiamen/S117/2015) and four representative Victoria lineage virus strains (B/Hong Kong/330/2001, B/Malaysia/2506/2004, B/Brisbane/60/2008, B/Xiamen/S136/2015) using ELISA (Figure 2A). In the eight strains tested, 12G6, 5A7, 2E01, 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 showed good binding activity with mean EC50 values of 55.85, 66.69, 74.52, 56.48, 59.46, and 68.01 ng/ml, respectively (Figure 2A). Antibody combinations 12G6 + 5A7 and 12G6 + 2E01 showed more virus-binding activity than 5A7 and 2E01 alone and similar binding activity compared to 12G6, even if it's not statistically significant. Notably, 12G6 + 5A7 showed the best viral binding activity among all three antibody combinations, while the control negative antibody showed no viral binding ability (Supporting Infornation: Figure S1A).
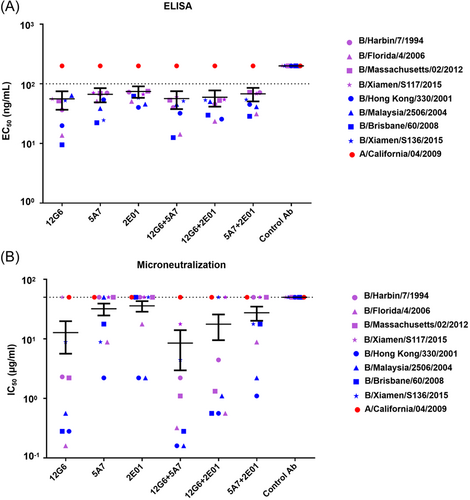
We then tested the neutralizing activity of 12G6, 5A7, 2E01, 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 against different representative influenza B viruses, we found that all antibodies exhibited virus-neutralizing activity against viral strains from different lineages, with mean IC50 values of 12.74, 31.95, 35.79, 8.48, 17.61, and 27.50 µg/ml, respectively (Figure 2B). Furthermore, the 12G6 + 5A7 antibody cocktail displayed the best overall neutralizing activity and 12G6 + 2E01 and 12G6 + 5A7 had lower IC50 values than 5A7 and 2E01. Among them, the comparison of antibody combination 12G6 + 5A7 and single antibodies 5A7 and 2E01 was statistically significant, while the comparison of other antibodies was not statistically significant (Supporting Infornation: Figure S1B). Together, these results suggest that cocktails of broadly neutralizing antibodies that recognize different epitopes of the influenza virus may exert synergistic antiviral effects.
3.3 Analysis of protective activities of the single and combined antibodies against different influenza B virus strains in mice
To further explore the protective effects of the different antibody cocktails against influenza B viruses in vivo, we first tested the protective effects of each antibody using a mouse model. Two MA influenza B virus strains from the Yamagata (B/Florida/4/2006) and Victoria (B/Hong Kong/330/2001) lineages, were administered intranasally to evaluate the prophylactic and therapeutic effects of different antibodies against lethal viral doses.
For the prophylactic experiments, single antibodies and antibody combinations (10 mg/kg) injected 24 h before viral infection protected mice against lethal doses of both viral lineages (Figure 3). The survival rate and body weight differed significantly between mice treated with all anti-influenza B antibodies and control IgG (p < 0.05; Figure 3A,B). In particular, the mean body weight was higher or only slightly decreased for mice in all influenza B antibody-treated groups compared to the original mean body weight (Figure 3C,D). Prophylactic treatment with any of the antibodies also reduced lung viral titers compared to the control animals, with 12G6 + 5A7 and 12G6 + 2E01-treated mice displaying the lowest lung viral titers (Figure 3E,F, Supporting Infornation: Figure S2A and B).
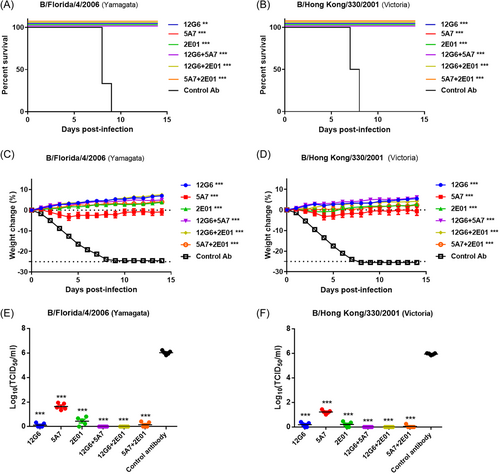
In the therapeutic assays (Figure 4), the antibodies were injected 24 h after infection with each virus. The 5A7 antibody partially protected mice from lethal challenges of influenza B virus strains from both lineages, whereas all other anti-influenza B antibodies (10 mg/kg) were able to completely protect mice infected with both influenza B lineages. Notably, the survival rates differed significantly between all anti-influenza B antibody-treated groups and the control IgG-treated group (p < 0.05; Figure 4A,B). In terms of weight recovery, 5A7 protected mice from lethal infection with Yamagata and Victoria lineage viruses, resulting in a moderate weight, while all the other anti-influenza B antibodies protected the mice from infection and resulted in an increased weight or slight body weight loss at the end of the study (Figure 4C,D). Moreover, we found that mice treated therapeutically with a single 10 mg/kg dose of any of the antibodies 1 day after infection had lower lung viral titers than the control animals, with the three antibody cocktails reducing lung viral titers more than the three single antibodies (Figure 4E,F, Supporting Infornation: Figure S3A and B).
3.4 Antibody cocktails provide better protection against influenza B virus than single antibodies when treatment is delayed in mice
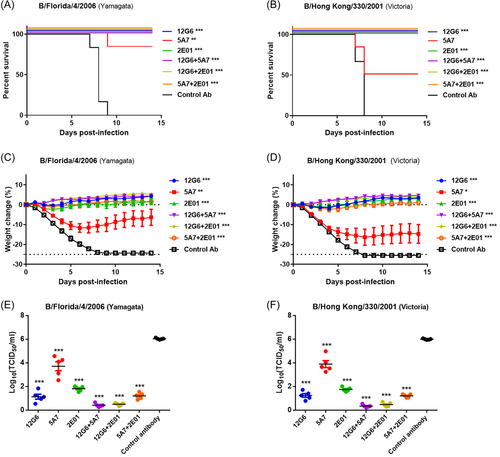
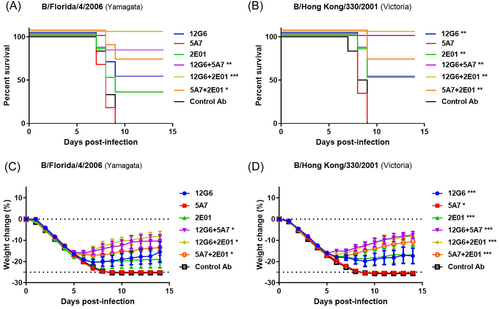
To explore the protective effect of antibody combinations in mice when the treatment was delayed after viral infection, we intravenously administered mice with the antibodies (10 mg/kg) 4 days after infection with the B/Florida/4/2006 (Yamagata) or B/Hong Kong/330/2001 (Victoria) strains and observed the survival rate and body weight of the mice in each group daily (Figure 5A,B). All animals in the control group died on the Ninth day after viral infection; however, treatment with 12G6, 5A7, 2E01, 12G6 + 5 A7, 12G6 + 2E01, and 5A7 + 2E01 protected the survival of 50%, 0%, 33%, 83%, 100%, and 67% of mice infected with the B/Florida/4/2006 strain, respectively. The survival rate of mice treated with the antibody cocktail was significantly better than that of mice treated with the negative control antibody, whereas the survival rate of mice treated with the single antibody did not differ significantly from the negative control (Figure 5A). For mice infected with the B/Hong Kong/330/2001 strain, treatment with 12G6, 5A7, 2E01, 12G6 + 5A7, 12G6 + 2E01, or 5A7 + 2E01 significantly improved the survival of 50%, 0%, 50%, 100%, 100%, and 67% of mice, respectively (Figure 5B). Notably, only the administration of the 12G6 + 2E01 antibody cocktail provided 100% protection against lethal doses of both lineages of the virus, and mice treated with the antibody cocktails had higher survival rates than those treated with single antibodies (Figure 5A,B). Weight monitoring revealed that delayed treatment with 12G6, 2E01, 12G6 + 5A7, 12G6 + 2E01, or 5A7 + 2E01 provided moderate protection against viral infections from both lineages and that treatment with the three antibody combinations was more effective than treatment with the single antibodies against both influenza B virus strains (Figure 5C,D).
3.5 Antiviral antibodies exert protective effects against the influenza B virus in ferrets
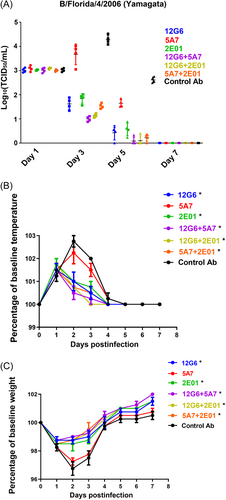
To further evaluate the potential protective effects of the anti-influenza B antibodies in vivo, we evaluated the therapeutic efficacy of single antibodies and their combinations (20 mg/kg) in ferrets 24 h after infection with the Yamagata lineage virus B/Florida/4/2006 animal-adapted strain (1 × 107 TCID50). Viral titers in ferret nasal washes, body temperatures, and weights were monitored on the indicated dates. On Days 1 and 7 after infection, viral titers in the nasal wash were similar in all treatment groups, with no significant differences between groups. On Days 3 and 5 after infection, the nasal wash viral titers were lower in ferrets treated with the antibody combinations than in those treated with the single antibodies, with 5A7 showing the worst antiviral efficacy and 12G6 + 5A7 showing the best (Figure 5A, Supporting Infornation: Figure S4A and B).
When we measured the temperatures of the experimental ferrets, we found that all antibodies were able to effectively reduce fever symptoms compared to the negative controls, except for 5A7. In particular, ferrets treated with the control and 5A7 antibodies displayed a significant increase in body temperature on Days 2 and 3 after viral infection, whereas those treated with 12G6, 2E01, 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 only exhibited a small increase in temperature on Days 2 and 3 after viral infection. In addition, ferrets treated with the three antibody combinations 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 had normal body temperatures on Day 3 after viral infection (Figure 6B and Supporting Infornation: Figure S5A and B). Moreover, infected animals administered C12G6, 2E01, 2G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 only experienced slight body weight loss; in contrast, considerable body weight loss was observed in control antibody and 5A7-treated animals. Ferrets treated with the three antibody combinations 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 return to normal weights on Day 4 after viral infection. (Figure 6C, Supporting Infornation: Figure S6A and B).
3.6 Antibody combinations can exert greater antiviral effects by enhancing ADCC
Finally, we investigated the specific mechanisms underlying the effects of the combined antibodies by performing ADCC assays. Previous studies have reported that the main antiviral mechanisms of broadly neutralizing antibodies against influenza virus NA include the inhibition of viral efflux and ADCC, while broadly neutralizing antibodies against HA may also possess these antiviral mechanisms.19, 22 Therefore, we evaluated the ADCC of 12G6, 5A7, 2E01, 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01 against the B/Florida/4/2006 (Yamagata) and B/Hong Kong/330/2001 (Victoria) influenza B virus strains (Figure 7A,B). When the target cells were infected with the B/Florida/4/2006 strain, C12G6 demonstrated no ADCC activity, 5A7, 2E01, 12G6+ 5A7, and 12G6 + 2E01 displayed higher ADCC activity, the values are 40%, 49.33%, 38%, and 48%, respectively. And 5A7 + 2E01 had the highest ADCC activity with a value of 66.67%, whereas the control antibody only showed background levels of activity (Figure 7A and Supporting Infornation: Figure S7). When the target cells were infected with the B/Hong Kong/330/2001 strain, 12G6 demonstrated comparably weaker ADCC activity, 5A7, 2E01, 12G6 + 5A7, and 12G6 + 2E01 displayed high ADCC activity, the values are 41.33%, 47.33%, 52.33%, and 62.67%, respectively. And 5A7 + 2E01 had the highest ADCC activity with a value of 64.33%, with all antibody combinations displaying higher ADCC activity than the single antibodies (Figure 7B and Supporting Infornation: Figure S8).

4 DISCUSSION
Influenza epidemics are a serious threat to global human health23 as the influenza virus has been associated with significant morbidity and mortality in children and the elderly.24, 25 However, currently available influenza vaccines and antiviral drugs display limited efficacy against the influenza virus26 and the proportion of drug-resistant influenza viruses is increasing.27, 28 It is therefore essential to develop new therapeutic drugs and vaccines.29 In this study, we explored whether different combinations of antibodies recognizing different antigenic epitopes on influenza viruses could exert synergistic antiviral effects compared to single antibodies. In particular, we examined the in vitro and in vivo efficacy of three different antibody combinations, 12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01, based on antibodies that recognize epitopes in the HA head region, HA stem region, and the NA active site of the influenza B virus.11, 17, 18 We revealed that: (1) 12G6, 5A7, and 2E01 antibodies recognize completely different antigenic epitopes on influenza B virus HA or NA and do not compete with each other for antigen binding; (2) Combining antibodies did not significantly enhance their binding activities but did significantly improve their neutralizing activities against influenza B viruses; (3) Antibody cocktails administered prophylactically significantly reduced the lung viral titers in mice compared to single antibodies. Similarly, the combined antibodies exerted better therapeutic effects than single antibodies, especially when treatment was delayed; (4) In ferrets, the antibody combinations also decreased the mean viral titers in nasal washes and restored a normal body temperature and weight more quickly than single antibodies; (5) The combined antibodies also displayed a higher ADCC than single antibodies. Since multiple pathways can be utilized to inhibit influenza viruses, the mechanism underlying viral neutralization by the combined antibodies and the avoidance of viral escape should be investigated further.
In this study, we demonstrated that antibody cocktails consisting of antibodies that recognize different antigenic epitopes against influenza viruses could be effective prophylactic and therapeutic agents. Notably, some antibody combinations allowed the survival of 100% of infected mice even after 4 days after infection with a lethal dose of influenza. These results suggest that neutralizing antibody cocktails that recognize multiple epitopes could be developed into novel drugs against influenza viruses. Consistently, previous studies have reported that cocktail therapies based on antibodies against two or three different epitopes could be developed into antiviral drugs against important human infectious diseases. For example, a mixture of two potent neutralizing antibodies that react with the Ebola virus glycoprotein has demonstrated high therapeutic efficacy in mice,30 while a mixture of two human antibodies was reported to synergistically inhibit the activity of the SARS-CoV-2 virus.16 It has also been shown that combining HIV antibodies that recognize different epitopes into bispecific or trispecific antibodies can greatly enhance their neutralization spectrum and neutralizing activity.31-33 Similarly, in vivo therapeutic studies have concluded that long-term administration with single antibodies cannot effectively fight HIV infection and that the efficacy of antibody cocktails should be evaluated.32 However, no relevant studies have evaluated the antiviral activity of antibody cocktails recognizing different antigenic epitopes against the influenza virus. Our in vitro and in vivo findings regarding the antiviral effects of antibody combinations recognizing different antigenic epitopes against the influenza B virus provide a basis for further studies of antibody cocktails against other influenza viruses or other human viruses.
Despite these important findings, this study has several limitations: (i) Only representative influenza B virus strains isolated between 1994 and 2015 were used to evaluate the characteristics of single and combination antibodies in this study. Therefore, the breadth and potency of these antibodies should be completely evaluated against influenza B virus strains isolated in recent years; (ii) The mechanisms underlying the action of the antibodies used in this study were only investigated using ADCC assays; (iii) Only one strain of influenza B virus B/Florida/4/2006 (Yamagata) was used in the ferret protection assays; (iv) Antibodies were not expressed as bispecific or trispecific antibodies to compare the antiviral activity of each antibody; and (v) The findings of this study require further validation in clinical trials. In the future, we will design bispecific and trispecific antibodies using representative influenza virus antibodies to simplify their composition and further evaluate their antiviral effects in vitro and in vivo. We will also perform similar studies using antibody cocktails against the influenza A virus to verify whether our findings are also applicable to neutralizing antibodies against the influenza A virus.
In conclusion, we evaluated the synergistic antiviral effects of broadly neutralizing antibody combinations (12G6 + 5A7, 12G6 + 2E01, and 5A7 + 2E01) against the influenza B virus in vitro and in vivo, and developed a set of evaluation models to confirm their synergistic effects. We demonstrated that these advantageous combinations could exert good therapeutic efficacy against the influenza B virus and may be able to counteract its continuous mutation. Together, our findings provide important avenues for the development of novel drugs and vaccines against influenza viruses.
AUTHOR CONTRIBUTIONS
Chenguang Shen, Yixin Chen, Wei Zhao, Baisheng Li, and Ningshao Xia contributed toward the experimental design. Chenguang Shen, Limin Zhang, Linlin Zhai, Yushan Jiang, Khrustalev Vladislav Victorovich, Khrustaleva Tatyana Aleksandrovna, and Minghui Yang contributed toward manuscript preparation. Mengjun Li, Yuelin Wang, Dong Huang, and Zhujun Zeng contributed toward virus isolation, titration, and sequencing analysis. Chenguang Shen, Zuning Ren, Yushan Jiang, Hua Cao, and Li Zhu contributed toward the establishment and production of antibodies. Li Zhu, Qinghua Wu, Weiwei Xiao, and Bao Zhang contributed toward in vitro antibody characterization. Linlin Zhai, Yushan Jiang, Limin Zhang, and Fuxiang Wang performed the animal experiments. Linlin Zhai, Yushan Jiang, and Limin Zhang contributed toward the ADCC assay. Chengsong Wan and Fuxiang Wang performed statistical analyses.
ACKNOWLEDGMENTS
We thank the NIAID Biodefense and Emerging Infections Research Resources Repository (BEI Resources) for providing some of the reagents used in this study. This study was supported by grants from the National Natural Science Foundation of China (grant numbers 81902058, 32170939, and 82111530302), the Guangdong Basic and Applied Basic Research Foundation (grant numbers 2020A1515010368 and 2022B1515020075), the Shenzhen Science and Technology Innovation Commission for Research and Development Project (grant number JCYJ20190809183205622). The Guangdong Science and Technology Program key projects (grant number 2021B1212030014), and the Basic Research Project of the Key Laboratory of Guangzhou (grant number 202102100001).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




