A Phase 2 open label study of ledipasvir/sofosbuvir for 12 weeks in subjects with hepatitis B virus infection
Abstract
Retrospective data showed that when we administered ledipasvir/sofosbuvir (LDV/SOF) to patients with hepatitis B and C coinfection, there was a modest reduction in hepatitis B surface antigen (HBsAg). Therefore, we hypothesize that similar HBsAg reduction can be seen in hepatitis B virus (HBV) monoinfected subjects. Primary and secondary efficacy endpoints are the decline in HBsAg and HBV DNA at Week 12 from baseline, respectively. We conducted an open-label Phase 2 pilot study to evaluate the safety, tolerability, and antiviral activity of LDV and/or SOF for HBV. Eligible subjects were either suppressed on antivirals (Group B) or inactive chronic HBV (Group A, C, D). Group A and B received LDV/SOF. Group C and D received SOF 400 mg and LDV 90 mg, respectively. All subjects completed the study, and all related adverse events (AEs) were mild. No discontinuations due to AEs or hepatitis flare occurred. At Week 12, HBsAg decline (log10 IU/ml) was similar between Group A (0.399) and B (0.400), less in Group C (0.207), and none in Group D, and there was HBV DNA decline in the inactive chronic HBV groups. LDV and SOF are safe and well tolerated when given to chronic hepatitis B subjects and have modest antiviral activity, particularly when given in combination.
1 INTRODUCTION
Hepatitis B virus (HBV) chronically infects over 296 million people worldwide with roughly 1.5 million new infections occurring each year.1 Chronic hepatitis B (CHB) remains endemic in several countries with continued high prevalence in multiple regions of Asia and Africa. Compared to the general population, people living with CHB have significantly higher rates of developing liver cirrhosis and hepatocellular carcinoma (HCC).
Hepatitis B surface antigen (HBsAg) loss has been associated with improvements in liver histology including reversal of cirrhosis, decreased risk of HCC, and prolonged survival. Current treatment guidelines have acknowledged the importance of HBsAg clearance in CHB.2, 3 An emerging theme is that HBsAg clearance is associated with definitive remission of the activity of CHB and an improved long-term outcome.3 Recent data show that the risk of HCC is lower if HBsAg clearance occurs before 50 years of age.4 Therefore, HBsAg loss is currently the primary goal of CHB therapy and is synonymous to a functional HBV cure.
Nucleos(t)ide analogues (NA) treatment is currently an important component in the management of active CHB, providing durable on-treatment suppression of viral replication and resulting in long-term clinical benefits with a reduced risk of liver complications. Although NAs are highly effective in suppressing HBV viral replication, the incidence of HBsAg loss during NA therapy is cumulative but low (ranging from 0% to 3% in the first year of NA therapy).5 Consequently, continuous long-term use is recommended for most patients. Furthermore, NA therapy rarely results in HBsAg seroconversion.6 Therefore, novel therapies that can enhance rates of HBsAg loss and subsequent seroconversion after a finite treatment course are needed.
With the advent of direct-acting antivirals (DAAs) for the treatment of HCV, patients coinfected with HBV and HCV were treated for their HCV with 12 weeks of fixed-dose combination (FDC) ledipasvir/sofosbuvir (LDV/SOF) 90/400 mg orally once daily. Samples from this study were retrospectively analyzed for quantitative HBsAg (qHBsAg).7 Interestingly, investigators observed a decline in qHBsAg levels during LDV/SOF treatment with a mean reduction of 0.14, 0.25, and 0.47 log10 IU/ml, at Weeks 1, 4, and 12, respectively.7 Overall, 37 of 111 (33%) subjects studied in this cohort had an HBsAg decline of ≥0.5 log10 IU/ml at 12 weeks on LDV/SOF.7 Moreover, in a separate study, Loggi et al.8 also found a similar case of an HBV-HCV coinfected patient that was treated with anti-HCV DAAs and observed an HBV DNA increase of more than 2 log but with simultaneous HBsAg decline, and subsequent HBsAg loss.
2 METHOD
2.1 Study design and selection of participants
We conducted a Phase 2, open-label, pilot study of LDV/SOF for 12 weeks in subjects with HBV infection (ClinicalTrials. gov NCT03312023). The study protocol was approved by the Institutional Review Board. The trial was carried out in accordance with the International Conference on Harmonisation of Good Clinical Practice and the US Code of Federal Regulations applicable to clinical studies.
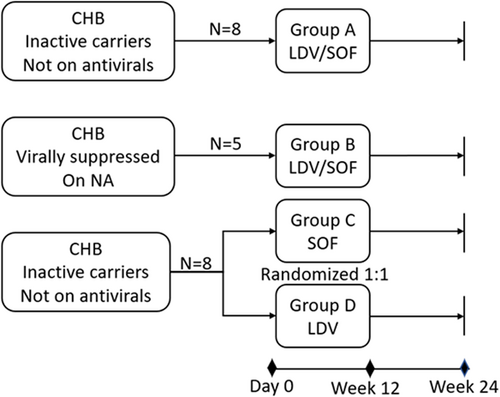
Adult participants were eligible to enroll if they had CHB, either in an inactive carrier state not requiring anti-HBV medication or were virally suppressed on only one NA. Participants were excluded if they were coinfected with HCV, hepatitis D, or human immunodeficiency virus, had known allergic reactions to LDV/SOF, evidence of cirrhosis or hepatic decompensation, HCC, on prohibited concomitant medications, or had illicit drug use or alcohol abuse within 12 months of screening. We assigned eligible participants to four different arms (Figure 1): Groups A and B—received 12 weeks of FDC LDV/SOF 400/90 mg once daily. Group A subjects were inactive carriers and therefore, did not require anti-HBV medication. Group B included subjects virally suppressed on NA and would remain on their respective NA during the study. Groups C and D enrolled inactive carriers not requiring anti-HBV medication and were randomized using block randomization in a 1:1 ratio to receive a 12-week daily dose of either SOF 400 mg once daily (Group C) or LDV 90 mg daily (Group D). They were randomized in blocks of two. This was done by the investigational drug services pharmacist utilizing a random number generator called Stattrek. A total of four blocks were generated with two volunteers in each randomized block.
2.2 Procedures
We utilized the US Food and Drug Administration-approved dosing and length of treatment for chronic hepatitis C treatment for this trial. Additionally, as declines in qHBsAg levels were seen when LDF/SOF was taken by those with HCV and HBV, our aim was to replicate the treatment to compare quantitative changes in a monoinfected population. LDV and SOF were taken orally regardless of food. As it is a fixed-dose combination, no dose adjustments were made. Study medications for the monotherapy arms used corresponding doses as those used in combination LDV/SOF therapy (i.e., 400 mg SOF or 90 mg LDV). Likewise, there were no dose adjustments made. We followed the participants until 12 weeks posttreatment with serial measurement of viral and safety labs performed on visit Day 0 (Baseline), Weeks 1, 2, 4, 8, 12, and posttreatment on Weeks 13, 16, and 24.
2.3 Outcomes
The primary efficacy endpoint was change in qHBsAg level at end of 12 weeks treatment compared to baseline and we used the paired t-test to determine the individual differences in HBsAg from baseline (Day 0) to end-of-treatment. Serum samples were collected and HBsAg was quantified by a chemiluminescence-based assay using the HBsAg CLIA kit (AutoBio Diagnostic Co. Ltd) following the manufacturer's instructions. HBsAg quantification was determined from a standard curve plotted with positive controls of known concentrations from 0 to 250 IU/ml. The kit has a sensitivity of less than 0.15 ng/ml. Diluted samples (1:1, 1:10, 1:100, 1:1000, and 1:10000 with phosphate buffered saline) from 100 μl serum were used for HBsAg quantification. Chemiluminescence was detected in the Synergy H1 plate reader (BioTek Instruments, Inc.). The secondary endpoint was to assess the antiviral activity of the study drug against HBV by measuring the decline in HBV DNA after 12 weeks of LDV and/or SOF from baseline. HBV DNA was quantified using commercially available cobas® HBV DNA quantitative real-time PCR for use on the cobas® 6800/8800 system. Safety and tolerability were evaluated by assessment of clinical laboratory tests, physical examination, and vital sign measurement at various time points during the study, as well as the documentation of adverse events (AEs). A treatment-emergent AE (TEAE) was defined as any new or worsening AE that was on or after the date of the first dose of the study drug up to the end of the study, that is, 12 weeks posttreatment. The study investigators graded the severity of each AE according to the NIH National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0,9 and monitored the toxicity data closely throughout the study for all enrolled subjects.
2.4 Statistical analyses
Study subjects' demographic and clinical characteristics, efficacy, and safety analyses were performed with the intention to treat population, which included all subjects who received at least one dose of the study drug. All subjects in the study were followed through the end of the study or censored at the date they were discontinued from the study. Appropriate descriptive measures were reported by group and by time. Both qHBsAg and HBV DNA and their respective log10 transformed values were described primarily using median and quartiles. Mean and standard deviations were also calculated. The change from baseline to Week 12 of log10 transformed qHBsAg and HBV DNA within groups was examined using Wilcoxon signed rank test. The decline from baseline was compared between groups using the Kruskal–Wallis test. We used IBM SPSS Statistics for Windows version 27.0.0.0 to conduct all statistical analyses, and GraphPad Prism version 9.1.1 to create all the graphs.
3 RESULTS
We enrolled a total of 17 individual subjects in the study. Group A enrollment occurred between April 2018 and October 2018, while the remaining Groups (B, C, and D) enrolled from October 2018 through February 2020. Five subjects who previously enrolled in Group A later enrolled in Groups C and D (two were randomized to Group C and three to Group D) with washout periods ranging from 189 to 366 days (median 303 days). One subject in Group A came in for Day 0 visit (signed treatment consent) but did not take the study drug and withdrew from the study thereafter. All the remaining subjects completed 12 weeks of study drug treatment and 12 weeks of posttreatment follow-up. Summary of subject enrollment and dispositions are included in Supporting information: Table 1.
| Group A | Group Ba | Group C | Group D | |
|---|---|---|---|---|
| Total enrolled (N) | 8 | 5 | 4 | 4 |
| Age (years) | ||||
| Mean ± SD | 47.3 ± 13.2 | 57.6 ± 11.4 | 43.5 ± 9.0 | 52.25 ± 7.8 |
| Median (Q1, Q3) | 47.0 (37.5, 52.75) | 58.0 (47.5, 67.5) | 39.5 (38.25, 52.75) | 53.0 (44.5, 59.25) |
| IQR | 15.25 | 20 | 14.5 | 14.75 |
| Range | 30–73 | 42–73 | 38–57 | 42–61 |
| Gender | ||||
| No. male | 7 (88%) | 3 (60%) | 2 (50%) | 4 (100%) |
| Ethnicity | ||||
| No. non-Hispanic | 8 (100%) | 5 (100%) | 4 (100%) | 4 (100%) |
| Raceb | ||||
| Black/African American | 4 (50%) | 2 (40%) | 3 (75%) | 3 (75%) |
| Asian | 3 (38%) | 3 (60%) | 0 | 0 |
| Caucasian | 1 (13%) | 0 | 1 (25%) | 1 (25%) |
| eAg negative, n (%) | 8 (100%) | 5 (100%) | 4 (100%) | 4 (100%) |
| ALT normal, n (%) | 8 (100%) | 5 (100%) | 3 (75%) | 4 (100%) |
| qHBsAg | ||||
| Mean ± SD, IU/ml | 6348.8 ± 5906.9 | 2417.5 ± 2228.4 | 3222.33 ± 4050.9 | 5332.4 ± 5403.5 |
| Median (Q1, Q3), IU/ml | 4706.64 (945.9, 11 924.9) | 1199.6 (617.3, 4826.8) | 1830.8 (360.9, 7475.3) | 3601.5 (1337.5, 11 058.4) |
| IQR | 10979 | 4209.5 | 7114.4 | 9720.9 |
| Mean ± SD in log10 IU/mlc | 3.5 ± 0.7 | 3.2 ± 0.5 | 3.1 ± 0.8 | 3.5 ± 0.5 |
| Median (Q1, Q3) in log10 IU/ml | 3.6 (2.9, 4.1) | 3.1 (2.7, 3.7) | 3.4 (2.6, 4.0) | 3.5 (3.1, 4.0) |
| IQR | 1.2 | 1 | 1.4 | 0.9 |
| Range, IU/ml | 172.1–15 489.6 | 257.2–4949.0 | 122.6–9105.1 | 1206.6–12 920.2 |
| Range in log10 IU/ml | 2.2, 4.2 | 2.4, 3.7 | 2.1, 4.0 | 3.1, 4.1 |
| HBV DNA | ||||
| Mean ± SD, IU/ml | 1163.8 ± 1363.7 | - | 1832.5 ± 2791.2 | 550.0 ± 356.6 |
| Median (Q1, Q3), IU/ml | 750.0 (152.5, 1642.5) | - | 555.0 (237.5, 4705.0) | 575.0 (207.5, 867.5) |
| IQR | 1490 | - | 4467.5 | 660.0 |
| Mean ± SD in log10 IU/mlc | 2.8 ± 0.6 | - | 2.9 ± 0.7 | 2.6 ± 0.5 |
| Median (Q1, Q3) in log10 IU/ml | 2.9 (2.2, 3.2) | - | 2.7 (2.4, 3.6) | 2.8 (2.2, 2.9) |
| IQR | 1.0 | - | 1.2 | 0.7 |
| Range, IU/m | 80–4170 | - | 220–6000 | 90–960 |
| Range in log10 IU/ml | 1.9, 3.6 | - | 2.3, 3.8 | 2.0, 3.0 |
- Abbreviations: HBV, hepatitis B virus; IQR, interquartile range; qHBsAg, quantitative HBsAg; SD, standard deviation.
- a All subjects in Group B had HBV DNA either <10 or undetectable at baseline and throughout the study.
- b Race categories: Caucasian (White), African American or African Descent (Black), Asian, and Others.
- c Mean in log10 IU/ml is calculated based on the mean (IU/ml) listed above it.
3.1 Baseline characteristics
Demographic and baseline characteristics of enrolled subjects per group are presented in Table 1. Subjects' age ranged from 30 to 73 years. The majority of enrolled subjects were of African American or African descent (12 of 21; 57%) and of the male gender (16 of 21; 76%). All subjects of all four groups had hepatitis B e antigen-negative status. Alanine aminotransferase (ALT) level was normal at baseline in all subjects except one. One subject randomized to Group C had an ALT of 48 IU/m (reference normal value of 0–44 IU/ml). The median baseline qHBsAg in IU/mL was highest in Group A (4706.64), followed by Group D (3601.5), Group C (1830.8), and lowest in Group B (1199.6). The median baseline HBV DNA in IU/ml was 750.0, 575.0, and 555.0 for Groups A, D, and C, respectively. Three of the five (60%) Group B subjects were on entecavir at baseline, while the remaining two were on tenofovir alafenamide. All subjects in Group B had HBV DNA levels at baseline either undetectable or under the lower limit of detection.
3.2 Efficacy results
All 13 subjects who received 12 weeks of LDV/SOF (Groups A and B) had qHBsAg decline in log10 IU/ml ranging from 0.091 to 0.769 at Week 12 compared to baseline. Groups A and B had similar qHBsAg log10 median decline in IU/ml at Week 12 (0.37 and 0.33, respectively), both of which were statistically significant declines when compared to baseline (p value 0.012 and 0.043, respectively). HBsAg decline in Group A was significantly different from Group D. Decline in qHBsAg was also seen in three of four (75%) of Group C subjects, and two of four (50%) of Group D subjects, although the median qHBsAg decline per group was less in Group C (0.06) and none in Group D. Table 2 presents a summary of efficacy endpoints.
| Group A | Group Ba | Group C | Group D | |
|---|---|---|---|---|
| Total enrolled (N) | 8 | 5 | 4 | 4 |
| qHBsAg, Week 12 | ||||
| Mean ± SD, IU/ml | 3057.5 ± 3493.2 | 937.3 ± 798.3 | 2161.2 ± 3177.3 | 7836.4 ± 9616.7 |
| Median (Q1, Q3), IU/ml | 1195.6 (497.6, 6006.1) | 841.6 (324.6, 1597.8) | 813.2 (235.4, 5435.0) | 4351.5 (1136.2, 18 021.6) |
| IQR | 5508.5 | 1273.2 | 5199.6 | 16885.4 |
| Mean ± SD in log10 IU/mlb | 3.1 ± 0.8 | 2.8 ± 0.6 | 2.9 ± 0.7 | 3.6 ± 0.6 |
| Median in log10 IU/ml | 3.1 (2.7, 3.8) | 2.9 (2.3, 3.2) | 2.9 (2.3, 3.6) | 3.5 (3.0, 4.2) |
| IQR | 1.1 | 1.0 | 1.3 | 1.2 |
| Range, IU/ml | 41.5–9579.5 | 66.2–2223.5 | 126.0–6892.4 | 993.7–21 649.0 |
| Range in log10 IU/ml | 1.6, 3.9 | 1.8, 3.3 | 2.10, 3.8 | 2.9, 4.3 |
| qHBsAg, Week 12 decline from baseline, log10 IU/ml | ||||
| Mean ± SD | 0.39 ± 0.2 | 0.4 ± 0.3 | 0.19 ± 0.3 | −0.05± 0.1 |
| Median (Q1, Q3) | 0.37 (0.26, 0.59) | 0.33 (0.16, 0.68) | 0.06 (−0.01, 0.53) | −0.04 (−0.20, 0.07) |
| p Value | 0.012c | 0.043c | 0.273c | 0.465c |
| IQR | 0.33 | 0.52 | 0.54 | 0.27 |
| Range | 0.10, 0.66 | 0.09, 0.77 | −0.01, 0.66 | −0.22, 0.08 |
| HBV DNA, Week 12 | ||||
| Mean ± SD, IU/ml | 567.4 ± 785.2 | - | 312.5 ± 310.1 | 870 ± 1355.6 |
| Median (Q1, Q3), IU/ml | 180.0 (40.0, 1227.5) | - | 305.0 (37.5, 595.0) | 275.0 (52.5, 2282.5) |
| IQR | 1187.5 | - | 557.5 | 2230 |
| Mean ± SD in log10 IU/mlb | 2.2 ± 0.8 | - | 2.2 ± 0.7 | 2.4 ± 0.8 |
| Median (Q1, Q3) in log10 IU/m | 2.3 (1.6, 3.1) | - | 2.3 (1.6, 2.8) | 2.2 (1.7, 3.3) |
| IQR | 1.5 | - | 1.2 | 1.6 |
| Range, IU/ml | 9–2100 | - | 30–610 | 50–2880 |
| Range in log10 IU/ml | 0.9, 3.3 | - | 1.5, 2.8 | 1.7, 3.5 |
| HBV DNA, Week 12 decline from baseline, log10 IU/ml | ||||
| Mean ± SD | 0.51 ± 0.6 | - | 0.68 ± 0.4 | 0.21 ± 0.8 |
| Median (Q1, Q3) | 0.52 (−0.09, 1.15) | - | 0.77 (0.27, 0.99) | 0.12 (−0.50, 1.01) |
| p Value | 0.093c | - | 0.068c | 0.465c |
| IQR | 1.24 | - | 0.72 | 1.51 |
| Range | −0.28, 1.36 | - | 0.17, 0.99 | −0.69, 1.28 |
- Abbreviations: HBV, hepatitis B virus; IQR, interquartile range; qHBsAg, quantitative HBsAg; SD, standard deviation.
- a All subjects in group B had HBV DNA either <10 or undetectable at baseline and throughout the study.
- b Mean in log10 IU/ml is calculated based on the mean (IU/ml) listed above it.
- c p Value using Wilcoxon Signed rank test. Bold p< 0.05 are considered significant.
HBV DNA median decline (in log10 IU/ml) at Week 12 was observed in the inactive chronic HBV groups—Group A (LDV/SOF) at 0.52, Group C (SOF) at 0.77, and Group D (LDV) at 0.12. Median declines at Week 12 from baseline in HBV DNA in all groups were not statistically significant.
The qHBsAg and HBV DNA, Week 12 decline from baseline, in log10 IU/ml, were compared between groups using the Kruskal–Wallis test with p < 0.05 considered to be significant. Supporting Information: Figure 1A illustrates the group comparison in qHBsAg log10 decline, showing a significant difference (p value of 0.027). Group A was significantly different compared to Group D. With regard to HBV DNA log10 decline, no significant difference was found between groups (Supporting Information: Figure 1B).
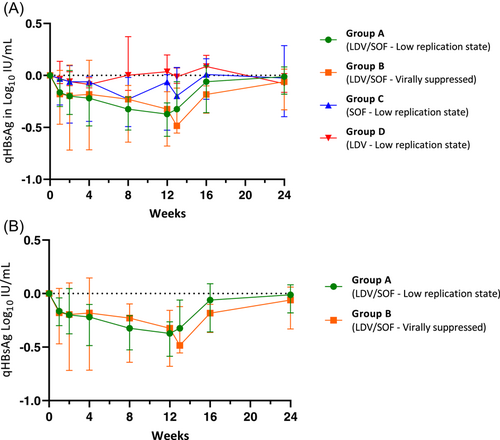
3.3 QHBsAg kinetics
The kinetics of qHBsAg in log10 IU/ml for 12 weeks of study drug treatment and after treatment are illustrated in Figure 2A (median with interquartile range [IQR]). Figure 2B compares qHBsAg log10 IU/ml over time between Group A and Group B. Both Group A and Group B had significant declines in qHBsAg from baseline at Week 8 and 12 of study drug treatment, with a notable return to baseline levels by 12 weeks posttreatment. No meaningful trends or declines in qHBsAg were observed in Group C or Group D.
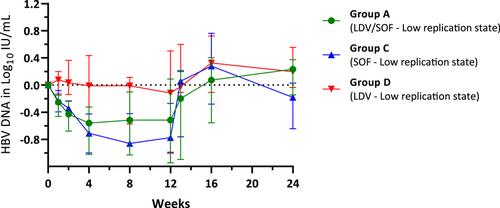
3.4 HBV DNA viral kinetics
The kinetics of HBV DNA in log10 IU/ml for 12 weeks of study drug treatment and after treatment are illustrated for groups that enrolled subjects with chronic HBV in a low replication state (Group A, C, and D) in Figure 3 (median with IQR). HBV DNA for Group B, virally suppressed subjects, were all either below lower limits of quantification or not detectable. Median HBV DNA log10 IU/ml decline from baseline was seen in Group C (SOF) and Group A (LDV/SOF), but not in Group D (LDV). The median decline in Group C was numerically more than in Group A but this was not statistically significant.
3.5 Safety results
Fourteen of 21 subjects (66%) treated with either combination LDV/SOF, or monotherapy of either SOF or LDV experienced at least one TEAE. There were 57 TEAEs reported, 16 of which (28%) were considered by the investigator to be related to treatment with the study drug (Supporting Information: Table 2). All but five of the TEAEs were mild (Grade 1) in severity and none led to discontinuation of the study drug. Headache was the most common TEAE, occurring in 7 of 21 subjects (33%), followed by upper respiratory tract infection in 4 of 21 subjects (19%), and anemia, fatigue, and nasal congestion in 2 of 21 subjects (10%) each. There were no serious AEs (SAEs) or hepatitis flares during the study. There were no clinically significant observations or trends noted in laboratory assessments, vital signs, or physical exam findings during the study.
4 DISCUSSION
In this single-center Phase 2 pilot study, CHB infected patients either with the low replicative state (inactive carriers) or virally suppressed on anti-HBV NA therapy showed a statistically significant decline in qHBsAg levels during the 12 weeks of therapy with combination LDV/SOF taken once daily. The mean HBsAg decline (Table 2) at Week 12 therapy with combination LDV/SOF (mean log10 IU/ml decline in the low replicative state group of 0.39 and NA-therapy suppressed group of 0.40) was similar to those seen in a prior HBV-HCV study whereby subjects were treated for HCV using 12 weeks of combination LDV/SOF and had a mean decline of 0.47 log10 IU/ml.7 Interestingly, the observed HBsAg levels gradual return to pretreatment baseline by 12 weeks posttreatment supports the hypothesis of a direct antiviral effect of LDV/SOF, albeit transient, as opposed to the unrelated natural decay of HBsAg.
We subsequently conducted a substudy (Groups C and D) and enrolled CHB patients with a low replicative state to determine if the antiviral effect seen in the combination arms can be replicated when SOF or LDV were given alone. What we found was neither SOF nor LDV when given alone for 12 weeks resulted in any significant decline in HBsAg levels during treatment. Although it is possible that prior exposure to LDV/SOF (five subjects from Group A were permitted to participate in Group C/D) may have affected this outcome. Moreover, sample sizes of Group C and D were smaller.
Hepatitis B virus DNA levels were also observed to decline during SOF and/or LDV therapy in the majority of subjects with CHB in a low replicative state. HBV DNA decline was similarly transient with the median (and mean) mostly returning to pretreatment baseline levels in about 1 week posttreatment. Nevertheless, the log declines per group were not statistically significant, which may have been limited by the small sample sizes.
As there was a prevailing concern of hepatitis B flare with HCV DAA therapy, such as LDV/SOF, as seen in the treatment of HBV-HCV coinfected patients, safety was a principal parameter assessed during the entire study—AEs were collected up to 12 weeks posttreatment. To minimize the risk of hepatitis B flare, we designed the study to enroll inactive carriers and those virally suppressed by anti-HBV NA therapy. In our study, LDV and SOF given either in combination or alone for 12 weeks were safe and well tolerated in patients chronically infected with hepatitis B. Side effects were comparable to studies with LDV/SOF when given for the treatment of HCV infection. All related AEs were mild with headaches being the most common. No subjects experienced SAEs, hepatitis B flare, or discontinued LDV/SOF due to AE. In addition, the use of LDV/SOF is well tolerated even when given to subjects on anti-HBV medications.
Uncovering the possible mechanism of action of LDV/SOF in regulating HBsAg is of great importance as our data indicated its potential role in HBsAg reduction. A combination of LDV/SOF appears effective in reducing HBsAg levels, while neither of them alone failed to do so. Ribonucleoside analogues are incorporated in the elongating viral RNA chain primarily by viral RNA-dependent RNA polymerases leading to chain termination, their incorporation by host DNA-dependent RNA polymerase (e.g., RNA Pol-II) is one possible explanation.10 Sofosbuvir, being a chain terminating ribonucleoside analog, may get incorporated in the pregenomic RNA during its synthesis by the host polymerases, and subsequently result in the reduction of both HBV DNA and HBsAg. However, the fact that the reduction of HBsAg is observed only in combination to LDV indicates LDV might play a crucial role in this process while the exact mechanism of action remains elusive. It is nevertheless, beyond the scope of this clinical study. This clinical study was designed as a “proof of concept” study to verify the therapeutic potential of these DAA drugs to control HBV infection, especially HBV-HBsAg reduction and HBV-DNA reduction. A further detailed in vitro study is warranted to focus on the possible mechanism of action.
This is a proof-of-concept study and therefore has several limitations. First and foremost is the small sample size, as such, intergroup and intragroup variations with regard to demographic and baseline virologic profiles exist including HBsAg and HBV DNA levels. Evaluation in a larger population would ameliorate the effect of variations in baseline qHBsAg and other virologic parameters. Another limitation is that five subjects from Group A (LDV/SOF) were later reenrolled in Groups C (SOF) and Group D (LDV). Although these subjects had HBsAg levels return to pretreatment levels at end of Group A participation, it is possible that antiviral effects seen in subsequent study participation were reduced due to prior exposure (e.g., perhaps due to resistance or partial resistance to the study drug). Lastly, the dose and duration of LDV/SOF used in this study were that of FDA approved for HCV treatment. It is unclear if administration of longer duration or higher dosing of LDV and/or SOF would result in a better antiviral effect or not, and therefore need to be assessed in future studies.
In summary, LDV and/or SOF, either in FDC or as monotherapy, are safe and well tolerated when given to chronic HBV-infected subjects. LDV/SOF has modest antiviral activity when given in combination. In addition to studying a larger sample size, further investigation is needed to understand the mechanisms and interactions that occur during LDV and SOF exposure in CHB patients and perhaps shed light on a potentially novel mechanism for HBV control.
AUTHOR CONTRIBUTIONS
Angie S. Price and Joel V. Chua drafted the manuscript. Joel V. Chua is the principal investigator that conducted the study. Joel V. Chua, Angie S. Price, and Amy K. Nelson cared for the patients and collected the data. Alip Ghosh conducted the quantitative HBsAg work. Angie S. Price, Shyamasundaran Kottilil, Amy K. Nelson, and Joel V. Chua conceptualized and designed the study. All authors reviewed and approved this manuscript.
ACKNOWLEDGMENTS
The authors had complete access to all data and total control over content and submission of the manuscript. We thank Nivya George for statistical support. This study was funded by Gilead Sciences Inc. (Grant # CO-US-337-4454).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the ethics committee of our University (Human Research Protection Office, University of Maryland) and fully complied with the Declaration of Helsinki and the Guideline for Good Clinical Practice. Informed consent was obtained from all subjects who participated in this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




