The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: A systematic review and meta-analysis
Abstract
Viral infections may increase the risk of developing type 1 diabetes (T1D), and recent reports suggest that Coronavirus Disease 2019 (COVID-19) might have increased the incidence of pediatric T1D and/or diabetic ketoacidosis (DKA). Therefore, this meta-analysis aims to estimate the risk of global pediatric new-onset T1D, DKA, and severe DKA before and after the COVID-19 pandemic. A systematic search of MEDLINE/PubMed, CINAHL, Scopus, and EMBASE was conducted for articles published up to March 2022. A random-effects meta-analysis was performed to compare the relative risk of T1D and DKA among pediatric patients with T1D between the COVID-19 pre-pandemic and pandemic periods. We also compared glucose and HbA1c values in children who were newly diagnosed with T1D before and after the COVID-19 pandemic. The global incidence rate of T1D in the 2019 period was 19.73 per 100 000 children and 32.39 per 100 000 in the 2020 period. Compared with pre-COVID-19 pandemic, the number of worldwide pediatric new-onset T1D, DKA, and severe DKA during the first year of the COVID-19 pandemic increased by 9.5%, 25%, and 19.5%, respectively. Compared with pre-COVID-19 pandemic levels, the median glucose, and HbA1c values in newly diagnosed T1D children after the COVID-19 pandemic increased by 6.43% and 6.42%, respectively. The COVID-19 pandemic has significantly increased the risk of global pediatric new-onset T1D, DKA, and severe DKA. Moreover, higher glucose and HbA1c values in newly diagnosed T1D children after the COVID-19 pandemic mandates targeted measures to raise public and physician awareness.
Abbreviations
-
- ACE2
-
- angiotensin converting enzyme 2
-
- CI
-
- confidence interval
-
- COVID-19
-
- coronavirus disease 2019
-
- DKA
-
- diabetic ketoacidosis
-
- ER
-
- event rate
-
- HbA1c
-
- glycosylated hemoglobin
-
- NOS
-
- Newcastle–Ottawa Scale
-
- PRISMA
-
- Preferred Reporting Items for Systematic Review and Meta-Analyses
-
- RR
-
- risk ratio
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- SMD
-
- standard mean difference
-
- T1D
-
- type 1 diabetes
1 INTRODUCTION
Type 1 diabetes (T1D) is an autoimmune disorder with a permissive genetic background that various environmental factors such as food chemicals, viral infections, and so forth act as autoimmune stimuli and eventually lead to the partial or complete destruction of pancreatic b-cells as a result of insulin deficiency.1 Numerous studies have reported the involvement of various viruses such as enteroviruses, Coxsackie B, Coxsackie A, Echo, cytomegalovirus, rotaviruses, retroviruses, and so forth in the pathogenesis of T1D.2-4 The causative agent of the current epidemic, coronavirus disease 2019 (COVID-19), is a positive-sense single-stranded RNA virus that acts on various organs in the body after entering human cells by binding to the angiotensin-converting enzyme 2 (ACE2). ACE2 binds to the membranes of various cells in the body, such as islets of Langerhans 5
Numerous types of diabetes including new-onset diabetes and metabolic complications such as diabetic ketoacidosis (DKA) and hyperosmolarity have been observed in patients with COVID-19.6 DKA is one of the most common and life-threatening acute complications of T1D. Ketoacidosis is the first manifestation of T1D or occurs when the need for insulin increases during illness or stress as well as when insulin intake decreases.7 Psychiatric disorders, stress, lower socioeconomic status, and elevated glycosylated hemoglobin (HbA1C) levels increase the risk of DKA.8 Infection is one of the most important predisposing factors for DKA in diabetic patients. Insufficiency of insulin injection also leads these patients to ketoacidosis.9 Diabetic ketoacidosis and its associated cerebral edema are the leading cause of hospitalization and mortality in diabetic children and adolescents and children under the age of 5 years are at higher risk for developing DKA.10 Despite the association between T1D and DKA with infection during COVID-19 epidemics, different findings have been reported. Gottesman et al. reported an increase in the incidence of new-onset T1D among US children during the COVID-19 pandemic.11 In contrast, Ho et al.12 reported no change and Rabbone et al.13 reported a decrease in T1D frequency. Despite different outcomes in the development of new-onset T1D, these studies have shown a significant increase in DKA and severe DKA in the diagnosis of diabetes in children and adolescents during the COVID-19 pandemic.11-14 Alfayez el al. recently published a systematic review and meta-analysis reported the risk of DKA and severe DKA during the COVID-19 pandemic versus the prior-to-COVID-19 period among pediatric patients with T1D and showed that risk of DKA and severe DKA increased significantly during the pandemic.15 Although, they did not perform any analysis on the number and rate of newly diagnosed children with T1D and the levels of glucose and HbA1c before and after the COVID-19 pandemic. Additionally, they did not identify all relevant studies in their search strategy and five relevant reports 16-20 were missed in their analyses. Moreover, they included a study by Danne et al. which does not report the risk of DKA and severe DKA among newly diagnosed patients.21 To date, several studies have reported childhood T1D as a new beginning in the COVID-19 pandemic. Given the dire consequences of childhood T1D, this study reviews and summarizes studies on the prevalence of childhood T1D during COVID-19.
2 METHODS
The present systematic review and meta-analysis were carried out in accordance with methodological guidelines from the Cochrane Handbook for Systematic Reviews.22 The findings of the present study were reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) statement (Online Supporting Material S1). 23
2.1 Search strategy
Relevant studies were systematically searched in electronic databases including MEDLINE/PubMed, CINAHL, Scopus, and EMBASE by two researchers (MA and MK) up to March 2022. The search strategy was as follows: (“severe acute respiratory syndrome coronavirus 2” or “novel coronavirus” or “COVID-19” or “2019-nCoV” or “SARS-CoV-2”) and (“type 1 diabetes mellitus” or “diabetes mellitus” or “juvenile onset diabetes” or “insulin-dependent diabetes” or “T1D” or “diabetic ketoacidosis” or “diabetic acidosis” or “acidosis”) (Online Supporting Material S2). Further, to find any other eligible articles, we searched all reference lists of included studies. Additionally, language restriction was not considered.
2.2 Eligibility criteria
The Eligibility criteria followed the PICOs question in the present systematic review and meta-analysis.24 We included studies that evaluated pediatrics' new-onset T1D during the COVID-19 pandemic in 2020 and during the same periods in 2019 which have reported at least one of the following outcomes: the number of children with new-onset T1D, the number of DKA among newly diagnosed children with T1D, and the number of severe DKA among newly diagnosed children with T1D. We also included studies that evaluated the level of hyperglycemia and HbA1c at T1D diagnosis before and after COVID-19 in newly identified children. Moreover, abstracts with insufficient data and studies with no reported sample size were excluded from the present meta-analysis.
2.3 Data extraction and quality assessment
First, all retrieved articles were screened by two investigators (M.A. and M.K.) in multiple levels of title, abstract, and full-text, and final studies that met the inclusion criteria were included. Second, the following data were extracted from eligible studies, where available: study design, country, age and gender, T1D diagnosis criteria, and relative outcomes. The quality of included studies was assessed using the Newcastle–Ottawa Scale (NOS).25 In all stages, discrepancies were solved by consensus with a third investigator (Sh.M.) before conducting a meta-analysis.
2.4 Statistical analyses
All meta-analyses were conducted using Comprehensive Meta-Analysis Software, version v. 2.0 (CMA, Biostat), and p value less than 0.05 was considered as significant. Dichotomous outcomes were pooled and expressed as logit event rate (ER), risk ratio (RR), and standard mean difference (SMD) with 95% confidence interval (CI).26 The pooled analysis results were classified based on study types into two categories, cohorts and cross-sectional and the pooled effect sizes were estimated using the random-effect model.27 Moreover, heterogeneity was calculated using Cochran's Q statistics and I2. Funnel plots with Egger weighted regression test were used for assessing the potential for publication bias. Finally, the overall pooled effect size of the respective outcomes was re-estimated by the one study that removed methods to perform sensitivity analysis.28
3 RESULTS
3.1 Study identification and characteristics
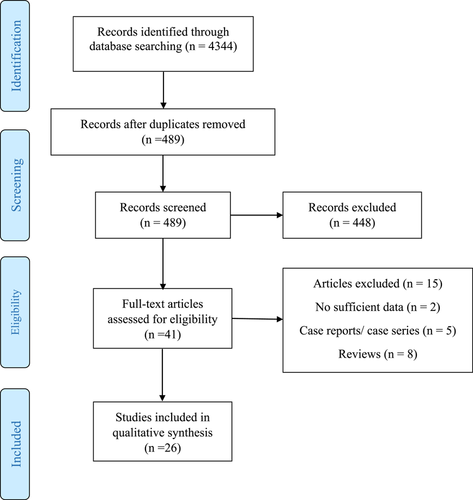
A total of 4344 potentially relevant articles were identified in our literature search. Five hundred and eighty-nine studies remained after removing duplicates. After screening titles and abstracts, 448 research articles were excluded. Of 41 obtained research articles, another 15 articles were excluded. Finally, 26 qualified articles met the eligibility criteria and were included in the meta-analysis (Figure 1). The characteristics of the included studies for meta-analysis are listed in Table 1. Data on newly diagnosed T1D among children during the COVID-19 pandemic period in all included studies were compared with those diagnosed during the same period in the previous year. The COVID-19 year in all included studies was defined as finding the first case of COVID-19 infected patient in the country. Publication ranged from 2020 to 2022 in most European countries, Saudi Arabia, Kuwait, Turkey, US, UK, Australia, Israel, Korea, and Canada. Data regarding the number of children infected by COVID-19 among all new-onset T1D were limited as 10 studies did not report any information about the number of diagnosed COVID-19 patients.12, 14, 18, 20, 30, 32, 37, 38, 40, 46 The number of COVID-19-positive cases in 16 remaining studies was as follows: one case in three studies,33, 35, 42 two cases in two studies,29, 36 four cases in two studies,17, 45 eight cases in four studies,13, 19, 31, 41 and no case in five studies.16, 34, 39, 43, 44 The worldwide incidence rate of diagnosis of T1D in the 2019 period was 19.73 per 100 000 children (18 years and younger) and 32.39 per 100 000 in the 2020 period. Compare with pre-COVID-19 pandemic, the number of worldwide pediatric new-onset T1D, DKA, and severe DKA during the first year of COVID-19 pandemic increased by 9.5%, 25%, and 19.5%, respectively. Compare with pre-COVID-19 pandemic, the median glucose (423.5 mg/dL vs. 397.9 mg/dL) and HbA1c values (12.26 ± 1.9% vs. 11.52 ± 2.3%) in newly diagnosed pediatric T1D children after COVID-19 pandemic were increased by 6.43% and 6.42%, respectively. All included studies were of moderate or high quality with NOS scores equal to or greater than 6 (Table 2). The designs of the included studies were cohort (n = 18) and cross-sectional (n = 8) and we performed a subgroup analysis based on different study types.
| Study | Design | Country | Age (year) | Gender, n (F) | Analyzed periods during the pandemic* | T1D diagnosis criteria | DKA diagnosis | Severe DKA diagnosis | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 2019, n | 2020, n | |||||||||
| Alaqeel et al. 202129 | Cohort | Saudi Arabia | 9.8 ± 0.2 | 154 (85) | March to June in 2020 | ADA | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 57 | 41 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 15 | 23 | |||||||
| Severe DKA | 4 | 7 | |||||||||
| Al-Abdulrazzaq et al. 202130 | Cohort | Kuwait | 8 ± 2.3 | 303 (153) | February to February of 2020 and 2021 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 303 | 324 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 113 | 166 | |||||||
| Severe DKA | 33 | 60 | |||||||||
| Atlas et al. 202116 | Cohort | Australia | NR | 58 (26) | February and May in 2020 | NR | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 89 | 58 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 41 | 30 | |||||||
| Severe DKA | 13 | 13 | |||||||||
| Boboc et al. 202131 | Cohort | Romania | 7.2 ± 0.2 | 147 (72) | March to February of 2020 and 2021 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 113 | 147 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 97 | 123 | |||||||
| Severe DKA | 33 | 41 | |||||||||
| Bogale et al. 202132 | Cohort | US | 9.2 ± 4.5 | 42 (19) | January to September in 2020 | NR | pH level < 7.3 | pH level < 7.1 | New-onset T1D | NR | 42 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 172 | 20 | |||||||
| Severe DKA | 123 | 13 | |||||||||
| Dilek et al. 202133 | Cross-sectional | Turkey | 10 ± 7.4 | 74 (39) | March to March of 2020 and 2021 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 46 | 74 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 27 | 68 | |||||||
| Severe DKA | 4 | 15 | |||||||||
| Dżygało et al. 202034 | Cohort | Poland | 9.9 ± 4.9 | 34 (12) | March to May in 2020 | WHO | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 52 | 34 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 29 | 18 | |||||||
| Severe DKA | 6 | 11 | |||||||||
| Goldman et al. 202235 | Cohort | Israel | 9.9 ± 2.8 | 146 (59) | March to June in 2020 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 113 | 146 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 44 | 85 | |||||||
| Severe DKA | 6 | 11 | |||||||||
| Gottesman et al. 2022 17 | Cross-sectional | US | 9.8 ± 0.2 | 187 (NR) | March to March of 2020 and 2021 | NR | NR | NR | New-onset T1D | 119 | 187 |
| DKA | 47 | 93 | |||||||||
| Severe DKA | NR | NR | |||||||||
| Hawkes et al. 202136 | Cohort | US | <18 | 73 (NR) | March to July in 2020 | ADA | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 92 | 73 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 33 | 35 | |||||||
| Severe DKA | 11 | 11 | |||||||||
| Herrero et al. 202218 | Cohort | Spain | 9.8 ± 1.4 | 37 (17) | January to January of 2020 and 2021 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 23 | 37 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 13 | 12 | |||||||
| Severe DKA | 5 | 2 | |||||||||
| Ho et al. 202112 | Cohort | Canada | 6–18 | 107 (61) | March to August in 2020 | DCCP | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 114 | 107 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 52 | 73 | |||||||
| Severe DKA | 3 | 8 | |||||||||
| Jacob et al. 202137 | Cross-sectional | Israel | 12 ± 2.7 | 86 (NR) | March to May in 2020 | ADA | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 80 | 86 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 31 | 46 | |||||||
| Severe DKA | 14 | 16 | |||||||||
| Kamrath et al. 202014 | Cohort | Germany | 6–18 | 532 (205) | March to May in 2020 | NR | NR | NR | New-onset T1D | 503 | 532 |
| DKA | 123 | 238 | |||||||||
| Severe DKA | 70 | 103 | |||||||||
| Kostopoulou et al. 202138 | Cohort | Greece | 8.3 ± 0.9 | 21 (12) | March to February of 2020 and 2021 | NR | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 17 | 21 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 6 | 14 | |||||||
| Severe DKA | 1 | 9 | |||||||||
| Lawrence et al. 202139 | Cohort | Australia | 8 ± 4.3 | 11 (8) | March to May in 2020 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 9 | 11 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 2 | 8 | |||||||
| Severe DKA | 1 | 5 | |||||||||
| Lee et al. 202140 | Cross-sectional | Korea | 12 ± 6.5 | 10 (9) | February to February of 2020 and 2021 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 10 | 10 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 4 | 6 | |||||||
| Severe DKA | 0 | 1 | |||||||||
| Mameli et al. 202141 | Cohort | Italy | 8.5 ± 4.2 | 256 (110) | March to December in 2020 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 231 | 256 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 65 | 91 | |||||||
| Severe DKA | 24 | 39 | |||||||||
| Marks et al. 202119 | Cohort | US | 10 ± 4.3 | 182 (81) | March to March of 2020 and 2021 | ADA | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 158 | 182 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 82 | 105 | |||||||
| Severe DKA | 27 | 51 | |||||||||
| McGlacken-Byrne et al. 202142 | Cross-sectional | UK | 10.3 ± 6.5 | 17 (8) | March to June in 2020 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 30 | 17 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 9 | 13 | |||||||
| Severe DKA | 3 | 8 | |||||||||
| Modarelli et al. 202243 | Cohort | US | 9.8 ± 0.2 | 46 (16) | April to March of 2020 and 2021 | ADA | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 31 | 46 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | NR | NR | |||||||
| Severe DKA | NR | NR | |||||||||
| Rabbone et al. 202013 | Cross-sectional | Italy | 0–14 | 160 (NR) | February to April in 2020 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 208 | 160 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 86 | 61 | |||||||
| Severe DKA | 31 | 27 | |||||||||
| Salmi et al. 202244 | Cross-sectional | Finland | 10 ± 2.3 | 20 (9) | April to October in 2020 | NR | NR | NR | New-onset T1D | 57 | 84 |
| DKA | NR | NR | |||||||||
| Severe DKA | 5 | 13 | |||||||||
| Sellers et al. 202120 | Cohort | Canada | 9.8 ± 0.2 | 260 (NR) | March to July in 2020 | NR | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 236 | 260 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | 86 | 143 | |||||||
| Severe DKA | 29 | 69 | |||||||||
| Unsworth et al. 202045 | Cohort | UK | 12 ± 6 | 30 (8) | March to June in 2020 | ISPAD | pH level < 7.3 | pH level < 7.1 | New-onset T1D | 15 | 30 |
| Bicarbonate level <15 mmol/L | Bicarbonate level <5 mmol/L | DKA | NR | NR | |||||||
| Severe DKA | NR | NR | |||||||||
| Vlad et al. 202146 | Cross-sectional | Romania | 0–14 | NR | January to June in 2020 | NR | NR | NR | New-onset T1D | 11.4** | 13.3** |
| DKA | NR | NR | |||||||||
| Severe DKA | NR | NR | |||||||||
- Abbreviations: ADA, American Diabetes Association; COVID-19, coronavirus disease 2019; DCCP, Diabetes Canada Clinical Practice; ISPAD, International Society of Paediatric and Adolescent diabetes; NR, Not reported; T1D, type 1 diabetes; WHO, World Health Organization.
- * The COVID-19 pandemic period in all included studies was compared with those diagnosed during the same period in the previous year.
- ** This study only reported the rate of incidence.
| Cohort study | Selection (4) | Comparability (2) | Outcome (3) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Representativeness of exposed cohort | Selection based on national registry data | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of study | Study control for diabetic risk factors | Adjustment of incidence rate for age | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |
| Alaqeel et al.29 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Al-Abdulrazzaq et al.30 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Atlas et al.16 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 5 |
| Boboc et al.31 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Bogale et al.32 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Dżygało et al.34 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Goldman et al.35 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Hawkes et al.36 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Herrero et al.18 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Ho et al.12 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Kamrath et al.14 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Kostopoulou et al.38 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Lawrence et al.39 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Mameli et al.41 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Marks et al.19 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Modarelli et al.43 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Sellers et al.20 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Unsworth et al. 45 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Cross-sectional study | Selection (5) | Comparability (2) | Outcome (3) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Representativeness of the sample | Sample size | Selection based on national registry data | Ascertainment of exposure | The subjects in different outcome groups are comparable, based on the study design or analysis. Confounding factors are controlled | Assessment of the outcome | Statistical test | |||
| Dilek et al.33 | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 8 | ||
| Gottesman et al.17 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
| Jacob et al.37 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 7 | ||
| Lee et al.40 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 7 | ||
| McGlacken-Byrne et al.42 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | ||
| Rabbone et al.13 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | ||
| Salmi et al.44 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | ||
| Vlad et al.46 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
3.2 The worldwide impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes
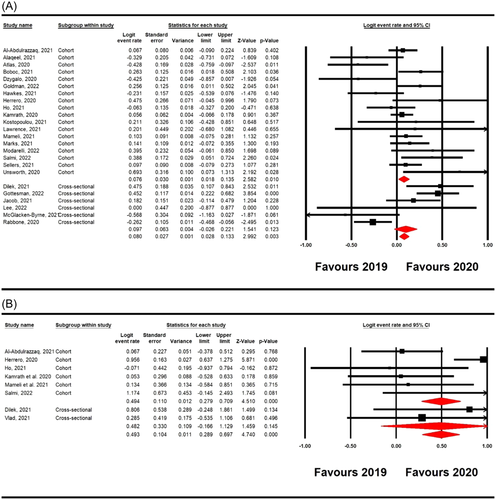
Twenty-four studies12-14, 16-20, 29-31, 33-45 involving 5671 new T1D patients (2706 new T1D patients in 2019 and 2965 new T1D patients in 2020) reported numbers of pediatric new-onset T1D before and after the COVID-19 pandemic. Overall, the COVID-19 pandemic was significantly associated with an increase in the number of worldwide pediatric newly diagnosed T1D (logit ER = 0.080, 95% confidence interval [CI] 0.028–0.133, p = 0.003; Figure 2A). Significant heterogeneity was observed among the included studies (I2 = 66%, p = 0.0001). According to the study types, the pooled main effect of COVID-19 pandemic in the number of worldwide pediatric newly diagnosed T1D in cohort and cross-sectional studies were logit ER, 0.076 (95% CI: 0.018–0.135; p = 0.010), and logit ER, 0.097 (95% CI: −0.026 to 0.221; p = 0.123), respectively. Additionally, eight studies12, 14, 18, 30, 33, 41, 44, 46 reported incidence rate of T1D before and after the COVID-19 pandemic. Overall pooled analysis showed that COVID-19 pandemic was significantly associated with an increase in incidence rate of diagnosis of T1D in children (overall: logit ER = 0.493, 95% CI 0.289–0.697, p = 0.001; cohorts: logit ER = 0.494, 95% CI 0.279–0.709, p = 0.0001; cross-sectionals: logit ER = 0.482, 95% CI −0.166 to 0.129, p = 0.145; Figure 2B).
3.3 The worldwide impact of COVID-19 pandemic on the risk incidence of pediatric diabetic ketoacidosis
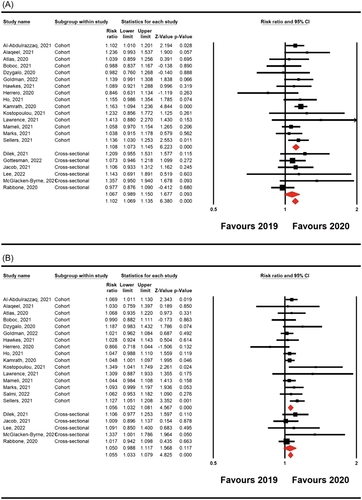
Twenty-one studies12-14, 16, 18-20, 29-42 involving 2648 new T1D patients with DKA and 979 new T1D patients with severe DKA (DKA: 1177 new cases in 2019 and 1471 new cases in 2020; severe DKA: 446 new cases in 2019 and 533 new cases in 2020) were included. The random-effect model showed that COVID-19 pandemic was associated with an elevation in the risk incidence of worldwide pediatric DKA and severe DKA compared with pre-COVID-19 period (RR = 0.064, 95% CI 0.043– 0.084, p = 0.0001, and RR, 0.049 (95% CI: 0.029–0.066; p = 0.0001, respectively; Figure 3). The values of I2 = 3% (p = 0.412) and I2 = 14% (p = 0.26) indicated that no significant heterogeneity exist in the included studies evaluating DKA and severe DKA. The pooled main effects were comparable for the different study designs: RR = 0.068, 95% CI: 0.045–0.091; p = 0.0001 (DKA in cohort studies), RR = 0.049, 95% CI: 0.028–0.070; p = 0.0001 (severe DKA in cohort studies), RR = 0.048, 95% CI: −0.002 to 0.093; p = 0.059 (DKA in cross-sectional studies), and RR = 0.049, 95% CI: −0.009 to 0.106; p = 0.096 (severe DKA in cross-sectional studies).
3.4 The worldwide impact of COVID-19 pandemic on the risk of increased hyperglycemia and HbA1c at T1D diagnosis
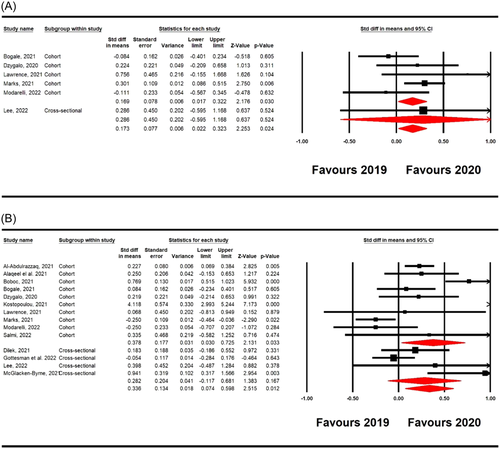
In total, six studies19, 32, 34, 39, 40, 43 included within this meta-analysis, which reported blood glucose and HbA1c levels in children who were newly diagnosed with T1D in 2019 and 2020. There were statistically significant associations between COVID-19 pandemic with elevation in blood glucose and HbA1c levels in pediatric newly diagnosed T1D compared with pre-COVID-19 period (SMD = 0.336, 95% CI 0.074–0.598, p = 0.012, and SMD = 0.173, 95% CI 0.022–0.323, p = 0.024, respectively; Figure 4). The SMDs observed for blood glucose in the cohort and cross-sectional studies were 0.169 (95% CI: 0.017–0.322, p = 0.030), and 0.286 (95% CI: −0.595 to 1.688, p = 0.524), respectively. Additionally, the SMDs observed for HbA1c in the cohort and cross-sectional studies were 0.378 (95% CI: 0.030–0.725, p = 0.033), and 0.282 (95% CI: −0.117 to 0.681, p = 0.167), respectively.
3.5 Sensitivity analysis and publication bias
The results of sensitivity analysis showed that the overall pooled estimates of the respective outcomes in all analyses obtained closely resembled preliminary associations. To further clarify the publication bias for the included studies, funnel plots suggested no noticeable bias in the studies of the present meta-analysis (Online Supporting Material S3). Further, Begg's correlation rank and Egger's regression did not show significant publication bias (Table 3)
| Risk factors | Effect measures | Number of study | Z-Value | p-Value | Effect size (95% CI) | Heterogeneity | Begg's test p-value | Egger's test p-value | |
|---|---|---|---|---|---|---|---|---|---|
| I2 | p value | ||||||||
| New-onset T1D | |||||||||
| Cohorts | Event rate | 18 | 2.582 | 0.010 | 0.076 (0.018–0.135) | 56% | 0.002 | 0.235 | 0.414 |
| Cross-sectionals | Event rate | 6 | 1.541 | 0.123 | 0.097 (−0.026 to 0.221) | 83% | 0.0001 | 0.425 | 0.468 |
| Overall | Event rate | 24 | 2.992 | 0.003 | 0.080 (0.028–0.133) | 66% | 0.0001 | 0.327 | 0.443 |
| T1D incidence rate | |||||||||
| Cohorts | Event rate | 6 | 4.510 | 0.0001 | 0.494 (0.279–0.709) | 71% | 0.004 | 0.286 | 0.198 |
| Cross-sectionals | Event rate | 2 | 1.459 | 0.145 | 0.482 (−0.166 to 0.129) | 0% | 0.444 | NA | NA |
| Overall | Event rate | 8 | 4.740 | 0.0001 | 0.493 (0.289–0.697) | 61% | 0.012 | 0.211 | 0.108 |
| Risk of DKA | |||||||||
| Cohorts | Risk ratio | 15 | 6.223 | 0.0001 | 1.108 (1.073–1.145) | 0% | 0.456 | 0.480 | 0.915 |
| Cross-sectionals | Risk ratio | 6 | 1.677 | 0.093 | 1.067 (0.989–1.150) | 9% | 0.356 | 0.132 | 0.112 |
| Overall | Risk ratio | 21 | 6.380 | 0.0001 | 1.102 (1.069–1.135) | 3% | 0.414 | 0.216 | 0.437 |
| Risk of Severe DKA | |||||||||
| Cohorts | Risk ratio | 16 | 4.567 | 0.0001 | 1.056 (1.032–1.081) | 14% | 0.289 | 0.471 | 0.449 |
| Cross-sectionals | Risk ratio | 5 | 1.568 | 0.117 | 1.050 (0.988–1.117) | 11% | 0.342 | 0.052 | 0.117 |
| Overall | Risk ratio | 21 | 4.825 | 0.0001 | 1.055 (0.033–1.079) | 9% | 0.334 | 0.183 | 0.209 |
| Risk of higher glucose | |||||||||
| Cohorts | SMD | 5 | 2.176 | 0.030 | 0.169 (0.017–0.322) | 42% | 0.136 | 1 | 0.970 |
| Cross-sectionals | SMD | 1 | 0.637 | 0.524 | 0.282 (−0.595 to 1.188) | 0% | 1 | NA | NA |
| Overall | SMD | 6 | 2.253 | 0.024 | 0.173 (0.022–0.323) | 29% | 0.216 | 0.573 | 0.945 |
| Risk of higher HbA1c | |||||||||
| Cohorts | SMD | 10 | 2.131 | 0.033 | 0.378 (0.030–0.725) | 89% | 0.0001 | 0.531 | 0.350 |
| Cross-sectionals | SMD | 4 | 1.383 | 0.167 | 0.282 (−0.117 to 0.681) | 67% | 0.025 | 0.174 | 0.172 |
| Overall | SMD | 14 | 2.515 | 0.012 | 0.336 (0.074–0.598) | 86% | 0.0001 | 0.139 | 0.182 |
- Abbreviations: CI, confidence interval; DKA, diabetic ketoacidosis; HbA1c, Glycosylated hemoglobin; SMD, standard mean difference; T1D, Type 1 diabetes.
4 DISCUSSION
In the present systematic review and meta-analysis, we performed a pooled analysis to evaluate and compare the effects of the first year of COVID-19 pandemic on the global incidence of T1D, DKA, hyperglycemia, and mean HbA1c levels in children. Based on the results of 26 eligible articles, the present meta-analysis shows that the global new-onset of childhood T1D rate and number have increased in 2020 compared with 2019. In addition, compared with pre-pandemic COVID-19 period, significant increases were observed in global DKA, severe DKA, blood glucose levels, and HbA1c levels in children.
Long-term complications of childhood-onset T1D have been considered as a main cause of death and cardiovascular-associated disease.47 More importantly, even before the onset of diabetes-related complications, young people with T1D are still at a higher risk of mortality.48 A systematic review of 13 articles assessing structural changes in the central nervous system in children and adolescents with diabetes concluded that repeated episodes of acute hyperglycemia, for example, DKA, are associated with detrimental structural changes in the brain.49 Additionally, acute diabetic complications including DKA and hyperglycemia were identified as leading causes of death before the age of 30 in a cohort study of 7871 childhood-onset T1D in Norway.50 Moreover, the Brecon cohort study of 3642 individuals in Wales showed that a near threefold excess mortality before age 30 which persists in individuals with young onset T1D occurred before age 15 years, and ketoacidosis was the most common cause of death in these patients.51 Further, a nationwide cohort study of 12 652 individuals in the Swedish pediatric diabetes quality registry from 2006 to 2014 showed that higher mean HbA1c during childhood was associated with higher diabetes-related premature mortality in young people (<30 years of age).52 Overall, these studies indicate that hyperglycemia, higher mean HbA1c, and DKA are associated with an increased risk of mortality in individuals with young onset T1D before the age of 30 years.
Given the accepted theory of the pathogenesis of T1D1 and the global increasing incidence of severe T1D, DKA, and severe DKA in children during the recent pandemic, it can be hypothesized that SARS-CoV-2 is probably a stimulus for the autoimmune system, especially for pancreatic autoimmunity, and the initiation of T1D. Therefore, this hypothesis can be a common cause between these two diseases, and raising awareness about this issue is recommended. Although, further research is needed to demonstrate this hypothesis
T1D is a multifactorial disease and in addition to environmental stimuli (food, stress, etc.), exposure to infectious agents such as viruses in genetically predisposed individuals can trigger the disease.53, 54 It has been shown that the RNA virus carrying COVID-19 may damage pancreatic β-cells.33, 55 Several hypotheses have been proposed for the association between COVID-19 and the higher incidence rate of T1D. Diabetes increases the risk of infections, including viral infections, due to its innate immunodeficiency on neutrophil chemotaxis phagocytosis and cellular immunity.56 Inflammatory markers of COVID-19 enter the cell through the binding of the COVID-19 spike protein using the enzyme ACE2. The mechanism of this process is a decrease in the expression of ACE2 induced by COVID-19, which eventually leads to cell damage, inflammation, and respiratory failure. Pancreatic β-cells are affected by the enzyme ACE2, and this direct link puts diabetics at greater risk for COVID-19 and finally the infection can cause new diabetes 57
It is essential that all nondiabetic patients (especially those at high risk for metabolic disease) be evaluated for the possibility of developing new-onset diabetes. Other factors such as unknown biological factors, avoidance, limited access or delay in seeking medical care, fear of seeing a doctor because of the risk of infection, and failure to recognize DKA symptoms, and stress may be involved in combating the COVID-19 pandemic. It is clearly worrying that T1D and DKA remain undiagnosed during the limited interaction of patients referred to healthcare centers. Another interesting finding in the data collected in this study was the higher HbA1c during the pandemic among the new T1D, which could be due to delays and limitations in medical care etc. Owing to rising T1D and DKA rates, public awareness of the symptoms of the disease in the public, improvement of telemedicine technology due to concerns about COVID-19 in hospitals and quarantine, warning to take the milder symptoms of the onset of new diabetes seriously is warranted
Results of the present meta-analysis must be interpreted in light of its limitations. First, the present systematic review and meta-analysis only covered the first wave of the COVID-19 pandemic and future studies should evaluate the number of childhood new-onset T1D in 2021 and 2022. Second, data regarding the number of children infected by COVID-19 among all new-onset T1D are limited and this make it difficult to attribute such an increase in T1D, DKA, and severe DKA to COVID-19 infection. Third, our findings should be interpreted with caution since our meta-analysis did not capture the unexpected confounders, time-varying exposure such as multifactorial environmental stimuli (i.e., food, stress, and outdoor environmental factor), and different ethnic effect. Finally, the criteria used for the T1D diagnosis varied between studies and should be consistent in future studies
5 CONCLUSION
The results of the present meta-analysis demonstrate a global significant increase in the incidence of childhood new-onset T1D, DKA, and severe DKA with elevated hyperglycemia and mean HbA1c levels at T1D diagnosis in the first year of the COVID-19 pandemic compared to pre-COVID-19 period. Due to this fact, physicians should consider this issue in all nondiabetic children and monitor their blood sugar and HbA1C when accepting them for proper and early management. Based on these findings, continuous and repeated educational diabetes awareness should be delivered to physicians, caregivers, and the public to improve health outcomes in the world and change trends in childhood T1D and DKA
AUTHOR CONTRIBUTIONS
Masoud Rahmati and Jae Il Shin developed the idea and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Masoud Rahmati and Maryam Keshvari ran the search strategy; Masoud Rahmati and Maryam Keshvari selected articles and extracted data. Masoud Rahmati evaluated the quality of the literature. Masoud Rahmati, Maryam Keshvari, Shahrzad Mirnasuri, Dong K Yon, Seung Won Lee, Jae Il Shin, and Lee Smith wrote the manuscript. All listed authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as supplementary information. The data are available by accessing the published studies listed in Table 1.