High levels of C-reactive protein-to-albumin ratio (CAR) are associated with a poor prognosis in patients with severe fever with thrombocytopenia syndrome in early stage
Zishuai Liu, Rongling Zhang, and Wei Zhou contributed equally to this work.
Abstract
C-reactive protein-to-albumin ratio (CAR) can be used to assess the prognosis of various diseases. This study aimed to evaluate the relationship between CAR on the prognosis of patients with severe fever with thrombocytopenia syndrome (SFTS). This study included 155 SFTS patients from the Public Health Clinical Center of Dalian from January to December 2021. They were divided into survival and deceased groups based on the clinical prognosis. The independent risk factors for poor prognosis of SFTS patients at an early stage were determined by Cox regression. The efficacy of CAR prediction was assessed by the receiver operating characteristic (ROC) curve. A total of 155 patients were included in this study, with an average age of 61.98± 11.70 years, including 77 males and 65 females. The mortality rate of the patients enrolled in this study was 14.19%. Multivariate Cox regression indicated that CAR (hazard ratio = 2.585, 95% confidence interval [CI] 1.405–4.753, p = 0.002) could be an independent predictor for prognosis in SFTS patients at an early stage. CAR had an AUC of 0.781 (95% CI, 0.665–0.898, p = 0.000), a cutoff value of 0.57, a sensitivity of 0.77, and a specificity of 0.80, with better predictive efficacy, compared to neutrophil-to-lymphocyte ratio (NLR). High levels of CAR are associated with poor prognosis in SFTS patients, and CAR can be used as an independent predictor for SFTS patients.
1 INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) is a novel tick-borne disease caused by a novel phlebovirus. SFTS was first reported in China in 20091 and has been successively reported in Korea,2 Japan,3 and Vietnam4 in recent years. SFTS has multiple transmission routes and is mainly contracted through tick bites. Over the last few years, relevant studies have shown that it can also be transmitted through interpersonal transmission (human to human).5
SFTS can cause a wide range of clinical manifestations, including hyperthermia, thrombocytopenia, leukopenia, gastrointestinal symptoms, unconsciousness, disseminated intravascular coagulopathy (DIC), multiorgan failure with a mortality rate of 11–30.1, 6-8 Due to its high lethality and potential to cause pandemic transmission, SFTS was classified as a national reporting disease in China in 2010 and was listed by the World Health Organization as one of the top 10 priority infectious diseases in the 2018 annual review of the Blueprint list.9 The pathogenic mechanism of SFTS is unclear, and there is no specific effective antiviral therapy.10 Therefore, early identification of severe patients and timely intervention are essential in treating of SFTS patients.
CAR is the ratio of C-reactive protein to albumin. Previous studies have shown that CAR can be used to assess sepsis11 and also as an essential indicator to assess the prognosis of noninfectious diseases such as oncology12-14 and cardiovascular disease.15, 16 CAR is effective in assessing the severity of viral infectious diseases.17 We aimed to investigate whether CAR could be used as an indicator to assess the poor prognosis of SFTS patients at an early stage and provide a reference basis for clinical judgment and treatment planning.
2 METHODS
2.1 Study design and patients' enrollment
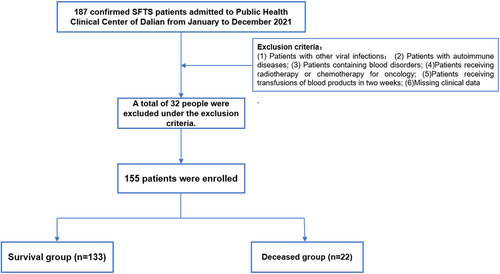
This retrospective analysis includes 155 SFTS patients from Public Health Clinical Center of Dalian from January to December 2021. All these patients should follow these diagnostic criteria: (1) Epidemiological history, (2) fever with temperature >37.5°C, (3) thrombocytopenia, and (4) positive serum nucleic acid test IgG and/or IgM antibody for SFTSV (New Bunyavirus), or specimens isolated to SFTSV. However, a total of 32 patients followed the excluded criteria: (1) Patients with other viral infections; (2) patients with autoimmune diseases; (3) patients containing blood disorders; (4) patients receiving radiotherapy or chemotherapy for oncology; (5) patients receiving transfusions of blood products in 2 weeks; and (6) missing clinical data (Figure 1).
This study was proposed following the principles of the Declaration of Helsinki and was approved by the Beijing Ditan Hospital, Capital Medical University (NO.DTEC-KY2022-022-01). Patients and their families were informed about this study and signed the relevant informed consent forms.
2.2 Data collection
We collect information related to patient demographics (gender, age, previous history, course, outcome), vital signs, general physical examination, including the nervous system, and laboratory tests (including routine blood tests, biochemical tests, coagulation, tissue damage, and inflammatory biomarkers). The observation endpoint of this study was defined as death or discharge.
2.3 Definition
Neurological physical examination includes the assessment of neurological signs and consciousness. A neurological sign was defined as at least one of the following change: muscle tension, involuntary movements, and nerve reflexes. Skin change was defined as at least one of the following signs: skin color changing, skin eruption, nodule. Hemorrhage was defined as at least one of the following symptoms: petechia, purpura, ecchymosis, hemoptysis, hematemesis, and melena.
2.4 Statistic analysis
The categorical variables are represented by percentages (n, %) and tested with the χ2 test or Fisher's exact test. Continuous variables that followed a normal distribution were expressed as mean () ± standard deviation (SD) and analyzed using the independent samples t test. Continuous variables that did not conform to a normal distribution were expressed as median (M) interquartile range (IQR) and analyzed using the Mann–Whitney U test for comparison. Univariate and multivariate COX regression analyses were applied to identify early independent risk factors for SFTS using the Stepwise method. The model was evaluated for prediction accuracy by receiver operating characteristic (ROC). Calculate the cut-off value based on the maximum Youden's index. Kaplan–Meier analysis was used to assess the cumulative survival of the groups with a lower and higher cutoff value of CAR. All data analyses were calculated by SPSS 26.0. The figure was made by GraphPad Prism 9.0.0. A two-sided p < 0.05 was regarded as statistical significance.
3 RESULT
3.1 Demographics and clinical characteristics of SFTS patients
Followed the inclusion and exclusion criteria, A total of 155 patients with a mean age was 61.98 ± 11.70 years in this study, including 77 males (77/155, 49.7%) and 78 females (78/155, 50.3%). The study included 22 deceased patients, with a fatality rate of 14.19%. Demographic information, clinical manifestation, and laboratory results are summarized in Tables 1–2.
| Variables | Total (n = 155) | Survival (n = 133) | Deceased (n = 22) | p value |
|---|---|---|---|---|
| Age (years) | 61.98 ± 11.70 | 60.43 ± 10.90 | 71.36 ± 12.27 | 0.000 |
| Male, n (%) | 77/155 (49.7) | 65/133 (48.9) | 12/22 (54.5) | 0.622 |
| Time from onset to admission (days) | 5.0 (4.0–7.0) | 5.0 (4.0–7.0) | 5.0 (4.0–7.3) | 0.795 |
| Hospitalization (days) | 8.0 (5.0–12.0) | 9.0 (6.0–13.0) | 4.0 (2.8–6.0) | 0.000 |
| Highest body temperature (°C) | 38.0 (37.0–39.0) | 38.1 (37.1–39.0) | 37.7 (36.7–38.8) | 0.165 |
| Bite by ticks, n (%) | 46/155 (29.7) | 41/133 (30.8) | 5/22 (22.7) | 0.441 |
| History, n (%) | ||||
| Diabetes | 14/155 (9.0) | 10/133 (7.5) | 4/22 (18.2) | 0.224 |
| Hypertensive disease | 21/155 (13.5) | 17/(12.8) | 4/22 (18.2) | 0.727 |
| CHD | 7/155 (4.5) | 5/133 (3.8) | 2/22 (9.1) | 0.260 |
| Cerebral infarction | 8/155 (5.2) | 7/133 (5.3) | 1/22 (4.5) | 1.000 |
| Liver disease | 10/155 (6.5) | 8/133 (6.0) | 2/22 (9) | 0.940 |
| Tumor related history | 4/155 (2.6) | 2/133 (1.5) | 2/22 (9.1) | 0.097 |
| kidney disease | 2/155 (1.3) | 1/133 (0.8) | 1/22 (4.5) | 0.265 |
| Other medical histories | 19/155 (12.3) | 16/133 (12.0) | 3/22 (13.6) | 1.000 |
| Symptoms | ||||
| Shiver | 50/155 (32.3) | 44/133 (33.1) | 6/22 (27.3) | 0.589 |
| Weak | 89/155 (57.4) | 80/133 (60.2) | 9/22 (40.9) | 0.091 |
| Chest distress | 6/155 (3.9) | 5/133 (3.8) | 1/22 (4.5) | 1.000 |
| Palpitation | 4/155 (2.6) | 3/133 (2.3) | 1/22 (4.5) | 0.461 |
| Muscular soreness | 56/155 (36.1) | 49/133 (36.8) | 7/22 (31.8) | 0.650 |
| Arthralgia | 40/155 (25.8) | 37/133 (27.8) | 3/22 (13.6) | 0.159 |
| Oliguria | 8/155 (5.2) | 8/133 (6.0) | 0 | 0.509 |
| Inappetence | 109/155 (70.3) | 99/133 (74.4) | 10/22 (45.5) | 0.006 |
| Nausea | 71/155 (45.8) | 67/133 (50.4) | 4/22 (18.2) | 0.005 |
| Vomiting | 17/155 (11.0) | 16/133 (12.0) | 1/22 (4.5) | 0.298 |
| Bloating | 19/155 (12.2) | 16/133 (12.0) | 3/22 (13.0) | 1.000 |
| Abdominal pain | 9/155 (5.8) | 8/133 (6.0) | 1/22 (4.5) | 1.000 |
| Diarrhea | 15/155 (9.7) | 11/133 (8.3) | 4/22 (18.2) | 0.286 |
| Neurological Symptoms | 18/155 (11.6) | 10/133 (7.5) | 8/22 (36.4) | 0.000 |
| Signs | 17/155 (11.0) | 12/133 (9.0) | 5/22 (22.7) | 0.124 |
| Skin changes | 17/155 (11.0) | 12/133 (9.0) | 5/22 (22.7) | 0.124 |
| Hemorrhage | 5/155 (3.2) | 2/133 (1.5) | 3/22 (13.6) | 0.021 |
| Pharyngeal swelling | 36/155 (23.2) | 31/133 (23.3) | 5/22 (22.7) | 0.952 |
| Chemosis | 6/155 (3.9) | 1/133 (0.8) | 5/22 (22.7) | 0.000 |
| Breath rough | 22/155 (14.2) | 18/133 (13.5) | 4/22 (18.2) | 0.803 |
| Rales | 6/155 (3.9) | 4/133 (3.0) | 2/22 (9.1) | 0.202 |
| Abdominal Tenderness | 11/155 (7.1) | 9/133 (6.8) | 2/22 (9.1) | 1.000 |
| Hepatosplenomegaly | 14/155 (9.0) | 14/133 (10.5) | 0 | 0.232 |
| Neurological signs | 25/155 (16.1) | 14/133 (10.5) | 11/22 (50.0) | 0.000 |
- Note: Continuous variable data are presented as mean (SD), median (IQR). Classified variable dates are presented as n/N (%), where N is the total number of patients with available data. p values comparing between the group of survival and the group of deceased.
- Abbreviations: CHD, coronary heart disease; IQR, interquartile range; SD, standard deviation; SFTS, severe fever with thrombocytopenia syndrome.
| Parameters | Total | Survival | Deceased | p value |
|---|---|---|---|---|
| (reference range) | (n = 155) | (n = 133) | (n = 22) | |
| WBC | 2.39 | 2.38 | 3.17 | 0.674 |
| (3.5–9.5 × 109/L) | (1.47–3.91) | (1.63–3.57) | (1.18–4.79) | |
| Neutrophils | 1.29 | 1.21 | 1.90 | 0.225 |
| (1.8–6.3 × 109/L) | (0.72–2.27) | (0.72–2.08) | (0.83–3.47) | |
| NEU% | 59.64 | 60.1 | 56.70 | 0.057 |
| (40–75) | (44.10–71.90) | (45.22–73.12) | (31.68–67.65) | |
| Lymphocytes | 0.66 | 0.67 | 0.44 | 0.017 |
| (1.1–3.2 × 109/L) | (0.40–1.24) | (0.43–1.26) | (0.28–0.86) | |
| LYM% | 32.24 | 32.90 | 20.60 | 0.006 |
| (20–50) | (19.40–43.74) | (20.38–45.28) | (14.53–34.50) | |
| Monocytes | 0.16 | 0.17 | 0.16 | 0.617 |
| (0.1–0.6 × 109/L) | (0.07–0.34) | (0.07–0.33) | (0.38–0.52) | |
| MON% | 6.74 | 6.77 | 6.50 | 0.866 |
| (3–10) | (3.70–10.70) | (3.48–10.38) | (1.73–17.50) | |
| RBC | 4.47 | 4.47 | 4.49 | 0.670 |
| (4.3–5.8 × 109/L) | (4.11–4.86) | (4.6–4.86) | (3.82–4.94) | |
| HGB | 137.00 | 137.00 | 137.00 | 0.540 |
| (130–175g/L) | (126.00–148.00) | (126.25–147.50) | (125.75–153.50) | |
| PLT | 58.00 | 60.50 | 43.5 | 0.001 |
| (125–350 × 109/L) | (38.00–82.00) | (41.00–88.75) | (21.75–59.25) | |
| LDH | 737.00 | 643.50 | 1185.50 | 0.002 |
| (80–285 U/L) | (401.00–1269) | (386.00–1091.92) | (666.23–2223.29) | |
| CK | 286.00 | 221.00 | 1273.75 | 0.000 |
| (0–190 U/L) | (102.00–890.12) | (91.50–631.50) | (415.43.00–2165.75) | |
| ALT | 90.00 | 83.00 | 122.65 | 0.120 |
| (9–50 U/L) | (49.00–168.00) | (47.50–153.30) | (75.75–304.28) | |
| AST | 157.00 | 134.95 | 315.50 | 0.001 |
| (15–40 U/L) | (73.00–325.00) | (65.25–266.50) | (124.25–767.15) | |
| TBIL | 9.70 | 9.20 | 12.35 | 0.007 |
| (2.0–20.4 μmol/L) | (7.00–13.30) | (6.80–12.75) | (9.39–16.80) | |
| DBIL | 4.00 | 3.80 | 6.40 | 0.000 |
| (0–6.8 μmol/L) | (2.70–5.89) | (2.60–5.20) | (4.52–10.56) | |
| ALB | 34.40 | 35.20 | 28.20 | 0.000 |
| (35–53 g/L) | (30.80–37.40) | (32.30–37.70) | (24.21–31.01) | |
| GLOB | 24.20 | 24.25 | 24.44 | 0.209 |
| (20–40 g/L) | (21.70–26.50) | (21.15–26.45) | (22.42–30.13) | |
| GGT | 51.00 | 49.00 | 97.00 | 0.009 |
| (11–49 U/L) | (27.00–110.00) | (24.00–96.00) | (36.43–172.26) | |
| ALP (40–150 U/L) | 67.00 (53.00–91.00) | 65.40 (53.00–85.00) | 69.8 (56.18–178.20) | 0.128 |
| GLU | 6.50 | 6.48 | 7.60 | 0.310 |
| (4.16–6.44 mmol/L) | (5.63–8.16) | (5.64–8.01) | (5.48–9.35) | |
| K+(3.5–5.1 mmol/L) | 3.45 ± 0.51 | 3.85 ± 0.51 | 3.83 ± 0.50 | 0.873 |
| Na+ | 133.70 | 133.65 | 133.42 | 0.984 |
| (136–146 mmol/L) | (131.00–136.3) | (130.90–139.21) | (131.00–141.82) | |
| Ca2+ | 1.97 | 1.98 | 1.93 | 0.010 |
| (2.2–2.55 mmol/L) | (1.93–2.05) | (1.93–2.05) | (1.83–2.00) | |
| PCT | 0.60 | 0.46 | 0.81 | 0.003 |
| (0–0.06 ng/ml) | (0.16–0.81) | (0.14–0.81) | (0.47–0.93) | |
| CRP | 5.04 | 3.78 | 16.20 | 0.000 |
| (5–10 mg/L) | (3.00–11.64) | (2.93–11.64) | (11.28–33.90) | |
| UREA | 5.18 | 4.98 | 7.12 | 0.000 |
| (1.7–8.3 mmol/L) | (4.22–7.10) | (4.10–6.50) | (5.93–11.66) | |
| CREA | 63.00 | 62.15 | 81.27 | 0.001 |
| (40–106 μmol/L) | (52.80–81.27) | (51.70–78.95) | (63.23–127.25) | |
| TT | 19.65 | 19.65 | 18.55 | 0.042 |
| (14–21 s) | (18.60–21.00) | (18.90–21.23) | (15.95–20.86) | |
| APTT | 43.70 | 42.25 | 53.20 | 0.060 |
| (11–14.5 s) | (35.60–48.61) | (35.68–48.61) | (34.75–67.88) | |
| PT | 13.20 | 13.70 | 11.05 | 0.010 |
| (11–14.5 s) | (12.10–14.74) | (12.60–14.74) | (10.70–11.70) |
- Note: Continuous variable data are presented as mean (SD), median (IQR). p values comparing between the group of survival and the group of deceased.
- Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransaminase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CK, creatine phosphokinase; CREA, creatinine; CRP, C-reactive protein; DBIL, direct bilirubin; GGT, γ-glutamyl transferase; GLOB, globulin; GLU, glucose; HGB, hemoglobin; IQR, interquartile range; LDH, lactate dehydrogenase; LYM, lymphocyte; MON, monocyte; NEU, neutrophil; PCT, procalcitonin; PLT, platelet; PT, prothrombin time; RBC, red blood cell; SD, standard deviation; SFTS, severe fever with thrombocytopenia syndrome; TBIL, total bilirubin; TT, thrombin time; WBC, white blood cell.
The enrolled patients were divided into a survival group and a deceased group according to clinical outcome. Compared to the survival group, patients in the deceased group were elder and had a shorter hospital stay, while no significant differences were seen in maximum body temperature, tick bite history, or previous history. Compared with the survival group, the patients in the deceased group had a higher frequency of chemosis, hemorrhage, and neurological manifestations.
All patients showed a decrease in platelet (PLT) counts. Patients who deceased had higher levels of lactate dehydrogenase (LDH), creatine phosphokinase (CK), alanine aminotransaminase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), procalcitonin (PCT), C-reactive protein (CRP), and lower lymphocyte (LYM), PLT, albumin (ALB), Ca2+ than survival patients. No significant differences were seen between the two groups for the remaining indicators.
3.2 Independent risk factors for deceased patients with SFTS
We explore independent risk factors for patients with SFTS to effectively identify patients with severe SFTS early and predict clinical outcomes. By performing univariate Cox regression analysis, we found that older age, hemorrhage, neurological signs/symptoms, decreases in LYM, PLT, CA, ALB, PT, and elevations in monocyte (MON)%, LDH, CK, AST, total bilirubin (TBIL), direct bilirubin (DBIL), GGT, alkaline phosphatase (ALP), urea, creatinine (CREA), procalcitonin (PCT), CRP, and CAR were considered significant factors for poor prognosis. variables with a p value less than 0.1 in the univariate Cox regression were brought in multivariate Cox regression with a stepwise method, the results showed that LYM (hazard ratio [HR] = 0.132, 95% confidence interval [CI] 0.033–0.523, p = 0.004), MON% (HR = 1.039, 95% CI 1.013–1.065, p = 0.003), CK (HR = 1.001, 95% CI 1.000–1.001, p = 0.000), ALP (HR = 1.006, 95% CI 1.002–1.011, p = 0.006), ALB (HR = 0.805, 95% CI 0.722–0.897, p = 0.000), CAR (HR = 2.585, 95% CI 1.405–4.753, p = 0.002), could be used as independent predictors of early death in SFTS patients (Table 3).
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Demographics and baseline characters | ||||
| Age | 1.101 (1.052–1.152) | 0.000 | ||
| Sex | 0.798 (0.344–1.850) | 0.599 | ||
| History | ||||
| Diabetes | 1.537 (0.206–11.474) | 0.675 | ||
| Hypertensive disease | 1.473 (0.497–4.363) | 0.485 | ||
| CHD | 2.171 (0.507–9.297) | 0.296 | ||
| Hemorrhage | 6.013 (1.770–20.433) | 0.004 | ||
| Neurological manifestation | 5.855 (2.442–14.036) | 0.000 | ||
| Laboratory findings | ||||
| WBC | 1.085 (0.928–1.268) | 0.306 | ||
| NEU | 1.013 (0.942–1.090) | 0.724 | ||
| NEU% | 1.024 (0.999–1.050) | 0.432 | ||
| LYM | 0.329 (0.111–0.977) | 0.045 | 0.132 (0.033–0.523) | 0.004 |
| LYM% | 0.955 (0.925–0.986) | 0.013 | ||
| MON | 1.076 (0.541–2.143) | 0.834 | ||
| MON% | 1.031 (1.007–1.056) | 0.011 | 1.039 (1.013–1.065) | 0.003 |
| RBC | 0.830 (0.498–1.385) | 0.476 | ||
| HGB | 1.002 (0.979–1.025) | 0.880 | ||
| PLT | 0.973 (0.956–0.991) | 0.004 | ||
| LDH | 1.001(1.000–1.001) | 0.000 | ||
| CK | 1.000 (1.000–1.000) | 0.000 | 1.001 (1.000–1.001) | 0.000 |
| ALT | 1.001 (0.998–1.004) | 0.609 | ||
| AST | 1.002 (1.001–1.003) | 0.000 | ||
| TBIL | 1.103 (1.037–1.174) | 0.002 | ||
| DBIL | 1.158 (1.087–1.234) | 0.000 | ||
| GGT | 1.003 (1.000–1.007) | 0.049 | ||
| ALP | 1.007 (1.003–1.011) | 0.001 | 1.006 (1.002–1.011) | 0.006 |
| ALB | 0.791 (0.727–0.862) | 0.000 | 0.805 (0.722–0.897) | 0.000 |
| GLOB | 1.078 (0.986–1.180) | 0.100 | ||
| GLU | 1.015 (0.967–1.066) | 0.552 | ||
| UREA | 1.029 (1.005–1.053) | 0.020 | ||
| CREA | 1.015 (1.008–1.022) | 0.000 | ||
| K+ | 0.853 (0.359–2.030) | 0.720 | ||
| NA+ | 1.022 (0.959–1.090) | 0.504 | ||
| Ca2+ | 0.008 (0.000–0.239) | 0.005 | ||
| TT | 1.021 (0.950–1.097) | 0.579 | ||
| APTT | 1.011 (1.002–1.020) | 0.150 | ||
| PT | 0.715 (0.541–0.944) | 0.018 | ||
| PCT | 1.173 (0.995–1.383) | 0.057 | ||
| CRP | 1.051 (1.029–1.073) | 0.000 | ||
| CAR | 4.505 (2.626–7.729) | 0.000 | 2.585 (1.405–4.753) | 0.002 |
- Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransaminase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CAR, C-reactive protein to albumin ratio; CHD, coronary heart disease; 95% CI, confidence interval; CK, creatine phosphokinase; CREA, creatinine; CRP, C-reactive protein; DBIL, direct bilirubin; GGT, γ-glutamyl transferase; GLOB, globulin; GLU, glucose; HGB, hemoglobin; HR, hazard ratio; IQR, interquartile range; LDH, lactate dehydrogenase; LYM, lymphocyte; MON, monocyte; NEU, neutrophil; PCT, procalcitonin; PLT, platelet; PT, prothrombin time; RBC, red blood cell; SD, standard deviation; SFTS, severe fever with thrombocytopenia syndrome; TBIL, total bilirubin; TT, thrombin time; WBC, white blood cell.
3.3 Diagnostic efficacy of CAR on clinical outcomes in patients with SFTS
Based on the results of multivariate Cox regression, CAR was used as a crucial factor for the early prediction of poor outcomes in SFTS patients. Predictive performance of CAR for prognosis in SFTS was assessed by using the AUC. the AUC for CAR was 0.781 (95% CI, 0.665–0.898, p = 0.000) (Table 4) (Figure 2A), and the AUC for NLR was 0.671 (95% CI, 0.556–0.786, p = 0.010). Compared with NLR, CAR had a better predictive performance. The cut-off value of CAR was calculated to be 0.57, with a sensitivity of 0.77 and a specificity of 0.80 (Table 4) (Figure 2B). According to Hosmer and Lemeshow Test (H-L Test) (p = 0.174), CAR has excellent predictive accuracy.
| Parameters | AUC | Cutoff values | Sensitivity | Specificity | 95% CI | p value | ||
|---|---|---|---|---|---|---|---|---|
| CAR | 0.781 | 0.57 | 0.77 | 0.80 | (0.665–0.898) | 0.000 | ||
| NLR | 0.671 | 0.30 | 0.96 | 0.35 | (0.556–0.786) | 0.010 | ||
- Note: The cutoff points were selected by maximizing the sum of sensitivity and specificity.
- Abbreviations: AUC, area under the ROC curve; CAR, C-reactive protein to albumin ratio; 95% CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; ROC, receiver operating characteristic; SFTS, severe fever with thrombocytopenia syndrome.

3.4 CAR is associated with poor prognosis in SFTS patients
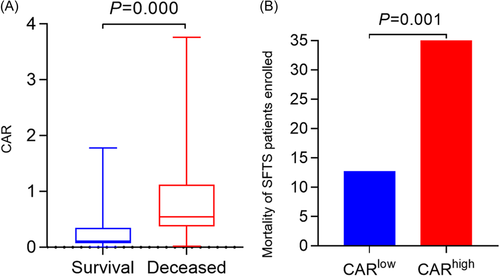
We found that patients in the deceased group had higher CAR than patients in the survival group [0.54 (0.37–1.12) vs. 0.11 (0.08–0.34) p = 0.000] (Figure 3A). According to CAR's cutoff value (0.57), all patients were divided into two groups CARlow and CARhigh. The fatality rate of SFTS patients in the CARhigh group was significantly higher than those in the CARlow group (12.78% vs. 40.91%, p = 0.001) (Figure 3B).
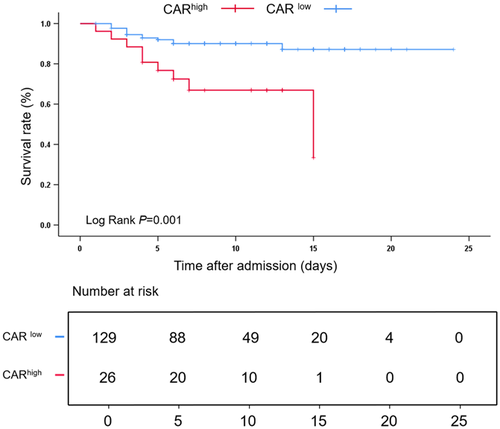
According to Kaplan–Meier survival curve analysis, SFTS patients with higher CARs had lower cumulative survival than those with lower CARs (Log-rank, p = 0.001) (Figure 4).
4 DISCUSSION
SFTS is a novel viral hemorrhagic fever disease with high mortality and wide transmission that is now attracting growing attention. However, there is no effective treatment. It is crucial to identify patients who need intensive treatment at admission. We, therefore, present for the availability of CAR to assess the prognosis of SFTS patients at an early stage.
In our cohort, patients mainly manifested with thrombocytopenia, liver dysfunction, elevated biomarkers of tissue damage, and a higher frequency of neurological-related manifestations, particularly in deceased patients, in parallel with previous studies.18
C-reactive protein is an inflammatory protein first discovered by Toilet and Francis in 1930, which is now widely used in assessing systemic inflammation and the status of infections.19 Previously, it was generally believed that elevated CRP was primarily seen associated with bacterial infections.20 Current studies have found that CRP can also be elevated in associated viral infections and can be used as an indicator to assess the disease.21, 22 It has also been reported that elevated CRP is an independent risk factor for patients with SFTS.23
Albumin is produced by the liver and accounts for 60% of serum protein.24 It is a vital nutrient for the body, maintains the stability of plasma colloid osmotic pressure, and is also one of the forms of transport of substances in blood circulation. In general, ALB is often used to assess nutritional status. However, current studies have shown that ALB is also closely related to the inflammatory response.25 The degree of decrease in ALB is positively correlated with inflammation. As with CRP, decreased albumin is also an independent risk factor for SFTS patients.26
A single indicator of CRP or ALB to assess disease has limitations. CAR is the ratio of CRP to ALB and was initially used to assess the prognostic assessment of patients in acute care.27 The current study found that CAR has a vital predictive efficacy of disease prognosis in viral infection,17 sepsis,28 severe burn injury,11 and even carcinoma.29, 30 We further found that CAR could be an independent risk factor for assessing poor prognosis in SFTS patients by COX regression and assessed the associated efficacy by AUC. We also introduced NLR, a standard inflammatory index, which effectively identifies patients at high risk for SFTS. In our study, CAR performs better in assessing poor prognosis than NLR. CRP and ALB are standard laboratory indices. These indicators are readily available, inexpensive, and reproducible, allowing us to identify high-risk patients early, intervene early, assess dynamically, and improve patient survival.
However, this study also has some limitations. First, this study had a limited sample from the same unit and was not validated using a new cohort. Secondly, we only tested the CAR at admission and lacked continuous measurements for assessing the disease. All these limitations need to be investigated further in the future.
5 CONCLUSION
High levels of CAR are associated with poor prognosis in SFTS patients, and CAR can be used as an independent predictor for SFTS patients.
AUTHOR CONTRIBUTIONS
Zishuai Liu, Rongling Zhang, Ruize Ma, and Wei Zhou collected, organized the clinical data, and prepared the manuscript. Leqiang Han, Zhe Zhao, Ziruo Ge, Xingxiang Ren, and Wei Zhang conducted statistical analysis. Zhihai Chen and Aijun Sun designed the study and reviewed the manuscript.
ACKNOWLEDGMENTS
Thanks to the staff from Public Health Clinical Center of Dalian for collecting data. Thanks to Shengqi Huang and Qinghuan Lin for supporting statistical software and data processing in this study. The study was supported by National Natural Science Foundation of China (No. 82072295).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon resonable request.