Association of gene polymorphisms of CD55 with susceptibility to and severity of hand, foot, and mouth disease caused by enterovirus 71 in the Han Chinese population
Abstract
Hand, foot, and mouth disease (HFMD) caused by enterovirus 71 (EV71) can lead to high morbidity and mortality, and genetic background plays an important role during the disease process. We investigated the association between the single-nucleotide polymorphism (SNP) rs2564978 of the CD55 gene and susceptibility to and severity of HFMD using the SNPs can multiple SNP typing methods. Soluble CD55 (sCD55) expression was significantly lower in the EV71 HFMD group than in the control group and lower in severe cases than in mild cases (P < .001). Moreover, CD55 rs2564978 (C vs T OR = 1.300, 95% CI, 1.120-1.509) was associated with the risk of EV71 infection, and genotype TC was related to the severity of the infection (TC vs TT OR = 4.523, 95% CI, 2.033-10.066). Our results suggest that sCD55 expression and the CD55 polymorphism rs2564978 may influence the susceptibility to and severity of EV71 infection.
Highlights
-
Serum CD55 expression was associated with susceptibility to and severity of HFMD caused by EV71.
-
The CD55 polymorphism rs2564978 may influence the susceptibility to and severity of EV71 infection.
1 INTRODUCTION
Hand, foot, and mouth disease (HFMD) is a common infectious disease that mainly affects infants and young children under 5 years of age.1, 2 Although most patients have a good prognosis, some develop neurological complications that lead to high mortality. HFMD is caused by a variety of enteroviruses, such as coxsackie virus A16 (CA16), enterovirus 71 (EV71), coxsackie virus A 6 (CA6), and coxsackie virus A 10 (CA10),3 of which EV71 has been recognized as the main pathogen in severe and fatal cases. Nonetheless, the pathogenesis of severe cases of HFMD remains elusive. The severity of the disease mainly depends on the pathogenicity of the virus and host immune response; indeed, clinical manifestations and outcomes vary widely among individuals, even among those infected by the same EV71 strain. This suggests that the host immune response plays a more important role than does the pathogenicity of the virus during the progression of the disease.
The innate immune system is the first line of defense against pathogens.4 In addition, the complement system is thought to be a key aspect in the innate immune system and acts as a bridge between innate and adaptive immunity. Under normal conditions, the complement system recognizes viruses and virus-infected cells and triggers effector pathways, resulting in neutralization of viruses and the killing of infected cells.5 CD556, 7 (or decay-accelerating factor), an important member of the regulator of complement activation protein family, is a known inhibitor of the complement system that suppresses C3 and C5 activation by preventing the formation of new C3 and C5 convertases and accelerating their decay.8 The two forms of CD55, a glycosyl phosphatidylinositol-anchored membrane form (mCD55) and a soluble form (sCD55),9 are found in a wide variety of human cells. Specifically, mCD55 is expressed on all blood cells, and sCD55 is widely distributed in body fluids.9 It has been reported that dysregulation of CD55 directly affects the progression of several diseases, including bullous pemphigoid,10 breast cancer,11 malaria,12 and H1N1 influenza.13
A study conducted in 2012 showed an association between CD55 polymorphisms and the severity of 2009 pandemic H1N1 influenza A virus (IAV) infection.13 Another study confirmed that the CD55 TT genotype is linked to the severity of H7N9/H1N1 pdm09 influenza in Chinese patients.14 Both of these studies suggest that the T/T genotype of the CD55 single-nucleotide polymorphism (SNP) rs2564978 is significantly associated with severe influenza virus infection; however, it is unclear whether it is associated with HFMD. In this study, we investigated the relationship between the CD55 gene polymorphism rs2564978 and susceptibility to and severity of HFMD in the Han Chinese population.
2 MATERIALS AND METHODS
2.1 Study participants
A total of 180 cases of HFMD caused by EV71 infection, as confirmed by a quantitative polymerase chain reaction, were identified from the Department of Infectious Disease in the Second Affiliated Hospital of Xi'an Jiaotong University or Xi'an Children's Hospital between 2014 and 2015. A total of 201 healthy children who underwent a health examination during the same period and had no history of HFMD were recruited as controls. All patients with HFMD were diagnosed in accordance with “Hand, Foot, and Mouth Disease Diagnosis and Treatment Guidelines (2010 version)” published by the National Health Commission of the People's Republic of China.15 Cases were classified as mild or severe depending on their manifestations and the guidelines mentioned above. Patients with the mild disease had rashes on their hands, feet, mouths, and buttocks, with some also having fever; patients with severe disease exhibited central nervous system complications such as meningitis, encephalitis, encephalomyelitis, or respiratory manifestations, especially pulmonary edema or circulatory problems. The subjects in both the mild and severe groups and in the control group included in this study belonged to the Han Chinese population.
2.2 Collection and preservation of specimens
We collected 1 mL of peripheral venous blood from the participants using a sterile, enzyme-free EDTA anticoagulation tube early in the morning and stored it at −20°C. Swab samples of the pharynx were also collected. We obtained 81 blood samples and 120 oral swab specimens from the healthy controls. Oral swab samples were collected using oral mucosal exfoliated cell collection rods and preserved at −20°C. The research protocol was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Xi'an Jiaotong University and Xi'an Children's Hospital. The guardians of all subjects signed informed consent forms.
2.3 DNA extraction and SNP genotyping
Genomic DNA was extracted from blood using Gentra Puregene Blood Kit and from throat swabs using Puregene Buccal Cell Kit (Germany) in accordance with the manufacturer's instructions. We used SNPscan Multiple SNP Typing Kit (Genesky Biotechnologies, Shanghai, China) to determine genotypes. The primer sequences used were as follows: rs2564978TR, AGAGACCTGGGGAAACAGGTTCAA; rs2564978CR, AGAGACCTGGGGAAACAGGTTCAG; and rs25649783R, TAACACAGTAGGGAGTGAACATTGTTCA. The Hardy-Weinberg equilibrium of the genotype distribution in the controls was calculated.
2.4 Determination of serum CD55 levels
Serum samples were collected from venous blood at room temperature and stored at −80°C. Serum CD55 levels were measured using a commercial enzyme-linked immunosorbent assay for human CD55 (Shanghai Jianglai Biotech, Shanghai, China) in accordance with the manufacturer's instructions.
2.5 Statistical analysis
Data were examined using SPSS 17.0 (IBM, Chicago, IL), and graphs were prepared by applying GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Genotype frequencies were analyzed for Hardy-Weinberg equilibrium. For continuous parameters, the mean ± SD was used to summarize the data, and the χ2 and Student t test were utilized to evaluate the importance of intergroup variations in demographic data and polymorphism frequencies. Median and quartile were used to summarize the data for counting variables, and the rank-sum test was applied to assess the significance of intergroup differences. Correlations are expressed as the odds ratio (OR) and 95% confidence interval (CI). The genotype and allele frequencies between the EV71 HFMD and control groups and between the mild and severe cases were compared using multivariate logistic regression analysis (corrected for age and gender). P < .05 was considered statistically significant.
3 RESULTS
3.1 Baseline demographics and characteristics
This study enrolled 180 patients (111 boys and 69 girls) with EV71 HFMD aged between 6 and 96 months (average 27.35 ± 17.02 months). Within this group, there were 72 cases of mild HFMD and 108 cases of severe HFMD. The patients in the control group (201 participants, including 119 boys and 82 girls) were between 8 and 72 months of age (average 25.45 ± 12.21 months). There were no significant differences in terms of age and sex between the HFMD and control groups or between the mild and severe cases (P > .05).
3.2 Relationship between the CD55 gene polymorphism rs2564978 and EV71 HFMD susceptibility
The genotype distributions, allele frequencies, and carriage frequencies of the control and EV71-infected groups are shown in Table 1. The CD55 polymorphism rs2564978 (C vs T: OR = 1.300, 95% CI, 1.120-1.509) was found to be associated with the risk of EV71 infection.
| Polymorphism | Genotype | Controls (%) (n = 201) | HFMD (%) (n = 180) | P | OR (95% CI) |
|---|---|---|---|---|---|
| rs2564978 | |||||
| TT | 70 (34.8) | 45 (25.0) | |||
| TC | 105 (52.2) | 88 (48.9) | .329 | 1.279 (0.780-2.095) | |
| CC | 26 (12.9) | 47 (26.1) | .000 | 1.864 (1.349-2.575) | |
| TC+CC | 131 (65.2) | 135 (75.0) | .034 | 1.651 (1.039-2.624) | |
| Allele | |||||
| T | 245 (60.9) | 178 (49.4) | |||
| C | 157 (39.1) | 182 (50.6) | .001 | 1.300 (1.120-1.509) |
- Note: Data are presented as n (%). Bold values emphasize the positive locus determined by statistical analysis.
- Abbreviations: 95% CI, 95% confidence interval; EV71, enterovirus 71; HFMD, hand, foot, and mouth disease; OR, odds ratio.
3.3 Relationship between CD55 gene polymorphism rs2564978 and EV71 HFMD severity
There was an obvious difference (P = .001) in the carriage frequency between the mild and severe cases. Indeed, greater severity of EV71 infection was associated with the TC genotype of CD55 rs2564978 (TC vs TT: OR = 4.523, 95% CI, 2.033-10.066) (Table 2).
| Polymorphism | Genotype | Mild cases (%) (n = 72) | Severe cases (%) (n = 108) | P | OR (95% CI) |
|---|---|---|---|---|---|
| rs2564978 | |||||
| TT | 26 (36.1) | 19 (17.6) | |||
| TC | 21 (29.2) | 67 (62.0) | .000 | 4.523 (2.033-10.066) | |
| CC | 25 (34.7) | 22 (20.4) | .679 | 1.094 (0.715-1.675) | |
| TC+CC | 46 (63.9) | 89 (82.4) | .008 | 2.581 (1.278-5.213) | |
| Allele | |||||
| T | 73 (50.7) | 105 (48.6) | |||
| C | 71 (49.3) | 111 (51.4) | .836 | 1.023 (0.827-1.266) |
- Note: Data are presented as n (%). Bold values emphasize the positive locus determined by statistical analysis.
- Abbreviations: 95% CI, 95% confidence interval; HFMD, hand, foot, and mouth disease; OR, odds ratio.
3.4 Relationship between serum CD55 levels and polymorphism
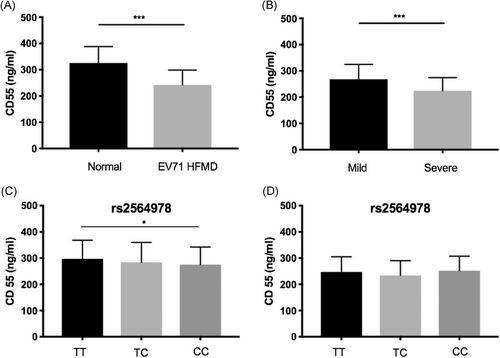
sCD55 levels were significantly lower in the EV71 HFMD group than in the control group (242.13 ± 56.57 vs 325.75 ± 62.42 ng/mL, P < .001) (Figure 1A); expression was also significantly lower in the severe cases than in the mild cases of HFMD (224.79 ± 49.65 vs 268.14 ± 56.66 ng/mL, P < .001; Figure 1B). Furthermore, in both the EV71 HFMD patients and controls, rs2564978 genotype CC was related to lower expression of CD55 compared with the TT genotype (275.21 ± 67.03 vs 296.77 ± 70.84 ng/mL, P < .05; Figure 1C). Conversely, no significant associations between rs2564978 and sCD55 levels were detected between the mild and severe cases (P > .05; Figure 1D).
3.5 Relationship between CD55 rs2564978 and clinical symptoms
The relationship between SNP rs2564978 and clinical symptoms is described in Table 3. Overall, SNP rs2564978 genotype TC was significantly associated with severe HFMD, a high fever, frequent confusion, white blood cell count > 15 × 109/L, glucose > 8.3 mmol/L, procalcitonin > 0.20 ng/mL, and previous Epstein-Barr virus infection (P < .05).
| Polymorphism | Genotype | |||
|---|---|---|---|---|
| rs2564978 | TT (n = 45) | TC (n = 88) | CC (n = 47) | P |
| Age, y (≤3) | 29 (64.4) | 65 (74.7) | 40 (85.1) | .074 |
| Male/female | 23/22 | 62/26 | 26/21 | .055 |
| Mild/severe | 23/22 | 21/67 | 25/22 | .000 |
| Fever | 45 (100) | 80 (90.9) | 38 (80.9) | .007 |
| Temperature (>39 ℃) | 4 (8.9) | 31 (35.2) | 9 (19.1) | .003 |
| Rash | 45 (100.0) | 88 (100.0) | 47 (100.0) | - |
| Frequent convulsions | 19 (42.2) | 67 (76.1) | 22 (46.8) | .000 |
| WBC > 15×109/L | 22 (48.9) | 64 (72.7) | 20 (42.6) | .001 |
| Glucose level (>8.3 mmol/L) | 19 (42.2) | 66 (75.0) | 24 (51.1) | .000 |
| PCT > 0.20 ng/mL | 17 (37.6) | 62 (70.5) | 20 (42.6) | .000 |
| EBV | 16 (35.6) | 62 (70.5) | 18 (38.3) | .000 |
| Deatha | 0 (0) | 2 (2.27) | 0 (0) | - |
- Note: Values are n or n (%), unless otherwise noted.
- Abbreviations: EBV, Epstein-Barr virus; PCT, procalcitonin; WBC, white blood cell count.
- a Causes of death were acute pulmonary edema, brainstem encephalitis, and circulatory failure.
4 DISCUSSION
Recent studies have demonstrated that host genetic background is related to susceptibility to a variety of viral infectious diseases, including influenza,16 respiratory syncytial virus infection,17 hepatitis B,18 and HFMD induced by EV71.19 In recent years, a large number of studies have focused on the relationship between SNPs and the susceptibility and severity of HFMD. For example, Yang et al20 showed that the IFN-γ+874A and IL-10-1082 A alleles are associated with susceptibility to EV71 encephalitis in Chinese patients. Li et al21 also found the IL-4-589 C allele to be a susceptibility factor in the development of EV71 disease in Chinese children. Our previous study reported that the RIG-I rs3739674 and RIG-I rs9695310 polymorphisms are associated with EV71 HFMD risk and that RIG-I rs3739674, TLR3 rs5743305, VDR rs11574129, and VDR rs739837 polymorphisms are associated with disease severity.22, 23 These findings support the important role of host genetic background in EV71 infection.
CD55 is an important complement regulatory protein and mostly functions by protecting autologous cells from complement damage and by disrupting the complement cascade.24 Recent studies also suggest a role for CD55 in signal transduction and modulation of T cell-mediated immune responses.25, 26 In addition, CD55 is the ligand for CD97,27 receptor for echovirus 7,28 and coxsackie viruses B1, B3, and B529; thus, it appears to be a target of immune evasion.30 Moreover, CD55 is upregulated in cells infected with various viruses, including influenza,13 parainfluenza,31 and hepatitis C viruses.32 According to Li et al,31 PIV5 derived from cells with higher CD55 levels is more resistant to complement-mediated neutralization in vitro than are viruses derived from control cells.31 Other studies have demonstrated that increased expression of CD55 decreases complement activation and inhibits complement-mediated neutralization and lysis of infected cells. In addition, expression of CD55 is downregulated in lymphocytes and red blood cells of patients with HIV infection,33 resulting in increased lymphocyte susceptibility to lysis by monoclonal antibodies directed by MHC class I antigen and its complements.34 Notably, CD55 functions as a double-edged sword and has both positive and negative effects in various diseases. In our study, we found that sCD55 levels were significantly lower in EV71 HFMD patients than in controls (P < .001) and that expression of sCD55 was significantly lower in severe cases than in mild ones (P < .001). This supports the fact that the level of sCD55 is associated with EV71 infection susceptibility and severity and that EV71 infection may downregulates CD55 expression in serum. This deficiency of CD55 may lead to hyperactivation of the complement system and heightened complement sensitivity, which in turn results in an increased inflammatory response. In addition to the functional role of the complement component, CD55 may modulate the induction of T cell immunity. For example, CD55 deficiency in mice significantly enhances the T cell response to active immunization, resulting in hypersecretion of interferon (IFN)-γ and interleukin (IL-2), as well as downregulation of the inhibitory cytokine IL-10 during antigen restimulation of lymphocytes in vivo.35 This result suggests that CD55 suppresses T cell immunity in vivo. The downregulation of sCD55 in the patients with EV71 infection in our study, especially severe cases, may also be associated with hyperactive T cell immunity, as previous studies have reported that EV71 infection causes increases in IFN-γ and IL-2.36, 37
The CD55 gene is located on chromosome 1q32 within a locus that encodes other regulators of complement activation.38 The rs2564978 SNP of CD55 resides in the minimal promoter region39 and is associated with promoter activity. In this study, we demonstrated that the CD55 rs2564978 polymorphism is significantly associated with EV71 infection; patients with the CC genotype (OR = 1.864, 95% CI, 1.349-2.575) or C allele (OR = 1.300, 95% CI, 1.120-1.509) were more susceptible to EV71 infection. In addition, the TC genotype was related to the severity of EV71 HFMD (OR = 4.523, 95% CI, 2.033-10.066), which is in line with the clinical variables of severe cases. We also found that rs2564978 genotype CC was related to lower levels of sCD55 compared with genotype TT in both the EV71 HFMD group and healthy controls (P < .05). Such lower levels of sCD55 result in reduced protection levels secondary to inappropriate or excessive activation of the complement system and indirectly enhance inflammation. We have clarified that CD55 polymorphisms and expression are related to EV71 infection and its progression. The results of our study are in contrast to those of Zhou et al,13 who reported that CD55 rs2564978 genotype TT was associated with severe IAV infection and that individuals with this genotype showed significantly lower levels of CD55 expression than did those with genotype CC.13 Although the genotypes involved in the two studies are different, the results suggest a functional variation in CD55 during virus infection. Perhaps because of its ubiquity and important role in the complement system, the potential functions of CD55 in HFMD are more complex than anticipated.
Our study has some limitations that need to be improved in subsequent studies on this topic. First, our sample size was relatively small, and the subjects were restricted to the Han Chinese population. Second, we did not study the correlation between CD55 rs2564978 genetic variation and CD55 expression levels in infected cells. Third, unlike genome-wide association studies, we evaluated only one SNP (rs2564978) and its functional variation in relation to the severity of EV71 infection. To conclude, we found sCD55 expression and the CD55 rs2564978 polymorphism to be associated with the susceptibility to and severity of HFMD in the Han Chinese population. Nonetheless, the determination of the exact mechanism of these effects requires further study.
ACKNOWLEDGMENTS
The authors thank the doctors of the Department of Infectious Diseases of Xi'an Children's Hospital and Second Affiliated Hospital of Xi'an Jiaotong University for their help in data collection and the Center for Human Genetics Research, Shanghai Genesky Bio-Tech for their technical assistance with genotyping. This study was supported by the National Natural Science Foundation of China (grant no. 81701632) and the Shannxi Province General Projects-Social Development (2017SF-154).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
S-SD conceptualized and designed the study. ML and Y-PL completed all experiments and drafted the manuscript. H-LD collected the clinical data, and M-QW, F-PW, and JW collected the clinical samples. W-JW performed the statistical analysis.




