HIV-1 subtype frequency in Northeast Brazil: A systematic review and meta-analysis
Abstract
Information on human immunodeficiency virus (HIV) molecular epidemiology is required to verify HIV/AIDS (acquired immune deficiency syndrome) epidemic dynamics in different regions, as well as provide support for response to antiretroviral therapy, transmission of resistance mutations, disease progression, and viral spread. The aim of this study was to conduct a systematic review and meta-analysis of the frequency of HIV-1 subtypes in Northeast Brazil. Seventy-six articles that refer to HIV-1 and its subtypes in the Northeast Brazil and published between 1 January 1999 and 31 August 2019 were identified. We included 27 articles for the qualitative synthesis, thus analyzing results from 4466 patients and 4298 genomic sequences. The results showed that subtypes B, F, and C and recombinant BF were responsible for 76% (IC95%: 71-80), 8% (IC95%: 5-11), 2% (IC95%: 2-3), and 7% (IC95%: 4-12) infections, respectively. The highest proportion of subtype B infections (82.2%) was observed in Piauí, while the subtype F had a high frequency in Pernambuco (23.4%). Bahia presented 11.6% of the proportion of recombinant BF. In addition, several recombinants such as AG, BC, BCF, and BD have been identified in the region. This is the first systematic review and meta-analysis on the HIV-1 subtype distribution in Northeast Brazil and has shown a high circulating viral diversity. Although subtype B is predominant in Brazil, a large frequency of non-B subtypes has also been found, which may have consequences for response to antiretroviral therapy, disease progression, and transmission. Thus, HIV molecular epidemiological data are essential for epidemic prevention and control strategies.
Research Highlights
-
A large number of studies were analyzed for only one region of a country (n = 27), with a total of 4298 HIV-1 sequences.
-
The Northeast region of Brazil, the second most populous in the country, has a variety of HIV-1 subtypes and Circulating Recombinants Forms.
-
There is a predominance of subtype B. However, in some states there are high proportions of other subtypes and recombinants.
1 INTRODUCTION
Human immunodeficiency virus and acquired immune deficiency syndrome (HIV/AIDS) epidemic remains a major public health problem in Brazil with more than 240 000 reported AIDS cases between 2007 and 2018, of which over 42 000 occurred in the Northeast region of the country.1 Most available epidemiological data are based on cross-sectional studies, which vary considerably in terms of local conditions, study populations, time, and methodology used. However, different methodologies have been used for epidemiological surveillance of HIV infection worldwide to assess and improve monitoring of trends within the epidemic. In this context, the genetic characteristics of HIV in each geographic region are different, mainly due to the high subtype distribution, host genetic factors, and selective antiretroviral therapy pressure.2 In addition, viral factors are known to play a role in the different patterns of AIDS progression, as co-circulation of HIV-1 subtypes. Thus, a unified subtype nomenclature system was established to describe the genetic variation created by mutation and recombination of the viral genome.3
Brazil is a continental country and its regions have unique demographic characteristics such as HIV-1 incidence. Northeast Brazil is composed of nine states (Alagoas, Bahia, Ceará, Maranhão, Paraiba, Pernambuco, Piauí, Rio Grande do Norte, and Sergipe) that together report 17% of the total AIDS cases identified in the country.1 HIV-1 molecular epidemiology data reveal that the Northeast epidemic is mainly caused by HIV-1 subtype B, followed by subtype F, in addition to the low prevalence of subtype C and circulating recombinant forms (CRF) such as BF.4, 5
However, there is a wide variation in the prevalence of HIV-1 subtypes in different states of Northeast Brazil.6 Thus, it is essential to determine a homogeneous profile of each region with studies on HIV-1 subtype frequencies in Northeast Brazil with considerable sampling so that the results reflect reality, providing information on the genetic and phylodynamic diversity of HIV. This study aimed to perform a systematic review and meta-analysis of HIV-1 frequency subtypes identified in Northeast Brazil.
2 MATERIALS AND METHODS
2.1 Systematic literature review
Scientific articles about HIV-1 subtypes and molecular epidemiology in Northeast Brazil were identified using the “STROME-ID (Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases),”7 a methodology applied to ascertain the content and quality of molecular epidemiological studies.
The following scientific article databases were searched: Cochrane Library, LILACS, PubMed, Scielo, and Web of Science, evaluating articles published between 1 January 1999 and 30 August 2019. Search terms (Mesh terms, headings, Emtree terms, or synonyms) included “HIV” or “HIV-1,” “subtype(s)” and “Brazil.” All search results for articles were organized in a central database for systematic review in the Mendeley reference manager (Elsevier Inc, New York, NY). Duplicate references were removed. The titles and abstracts of the articles obtained from the systematic review were examined and the full texts of those meeting the eligibility criteria were evaluated.
Studies identifying HIV-1 molecular epidemiology/subtypes from patients in the north-eastern states of Brazil were included as they provide data on HIV-1 genomic sequencing and/or viral subtyping methodologies. Methods for determining HIV-1 subtypes included genotyping (sequencing) of any virus genomic region (gal, pol, env, or other sequences) followed by subtyping using available online algorithms or molecular phylogeny.
Articles published in English, Spanish, or Portuguese within 1999 and 2019 were considered. Data from original articles or short communications were considered and those from case series or review articles were not. In the case of studies using a common database, only the original study (oldest) that used the data set was considered. “Grey literature,” such as theses, dissertations, monographs, and conference abstracts were not considered, as it was not possible to accurately stipulate whether the reported data were redundant to those reported in published articles.
2.2 Data extraction
Data regarding title, author, year of publication, location, study design, population characteristics, sample size, TCD4 and lymphocyte count average, viral load average, data collection period, blood sample collection period, HIV-1 genomic region analyzed, HIV-1 subtyping methods, and viral subtype frequency were extracted from the articles considered. The primary outcome was the frequency of HIV-1 subtypes, designated as A, B, C, D, F, G, H, J, and K and CRFs and unique recombinant forms (URFs). No patient identification information was retrieved, and all studies considered were approved by the respective Ethics Committees and/or submitted patient consents.
2.3 Data analysis and meta-analysis
Meta-analysis was performed using the R program Meta package (version 3.4.3). Heterogeneity among different populations was quantified by I2 and t2 values, according to the DerSimonian and Laird methods.8 Cochran's Q test, with “n − 1” degrees of freedom (where “n” is the number of studies), was used to assess whether heterogeneity was significantly different from zero. If the heterogeneity between studies was considered significant, the random-effects model was applied instead of the fixed-effects model.9, 10 According to I2 values, heterogeneity was considered low (≤25%), moderate (>25 and ≤50%), high (>50 and ≤75%), and very high (>75 and ≤100%). The significance level (α) of the Cochran Q test was set at 0.10.10 Meta-analysis resulted in the frequency of subtypes with a 95% confidence interval (95% CI). In the R software interface (version 3.4.3), the package meta: library(meta) was installed, then we loaded the table with the data for each subtype separately: table = read.table (“table.txt,” header = T, sep = “\ t”), then we identify the Author and Year headings of the table: table$studlab = paste (table$Author, c$Year). The heterogeneity test was performed using the metaprop command and the most appropriate effects model for the data was analyzed: table2 = metaprop (C,total, dat = c, sm = “PLOGIT,” studlab, predict = FALSE, level.predict = 0.95, hakn = FALSE, comb.fixed = TRUE, comb.random = FALSE, level.comb = 0.95, method.tau = “DL,” method.bias = “linreg,” warn = TRUE). The forest plot was then generated: forest (table2, comb.fixed = TRUE, comb.random = FALSE). The forest plot was converted to the TIFF file format: tiff (file = “table.tiff,” bg = “white,” width = 2896, height = 1700, units = “px,” res = 300). Then, the data titles were added to the forest plot: forest (c2, comb.fixed = TRUE, comb.random = FALSE, leftlabs = c(“Authors and Year,” “Subtype C,” “Total”)) dev.Off(). Thus the final forest plot was stored and the frequency of the subtype was identified.
3 RESULTS
3.1 Distribution of HIV-1 subtypes and recombinants in Northeast Brazil
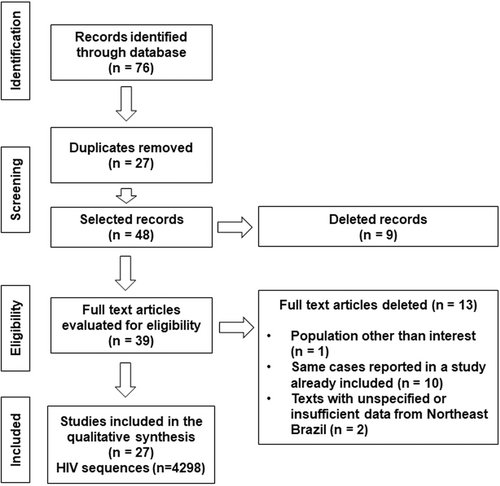
We identified 76 publications in the Cochrane Library, Lilacs, Medline (PUBMED), Scielo, and Web of Science. After excluding the duplicates (27), 49 articles were selected for reading the titles and abstracts. Nine articles were further excluded because they reported data from geographic regions other than Northeast Brazil. Out of the 39 articles selected, data were extracted from 27 articles published between 1999 and 2019, analyzing 4298 HIV genomic sequences in total (Figure 1).
Among the articles identified in the systematic review (n = 27), most reported data from the states of Bahia (n = 15), Pernambuco (n = 07), Ceará (n = 5), and Piauí (n = 04). The HIV-1 genomic regions from patients were sequenced between 1998 and 2016 and the study sample sizes ranged from 11 to 1300 patients. HIV-1 subtype B was most frequently reported, followed by subtypes F and C. Among the recombinants, BF was the most commonly reported recombinant. The subtype B had a frequency higher than 65%, which is 23 studies (from a total of 27). However, the lowest frequencies (54.6% and 54.5%) were observed in Bahia and Pernambuco, respectively. The subtype F was highly reported in Pernambuco, the frequency ranging between 22.6% and 39.8%. In addition, BF recombinants showed high dispersion in the north-eastern region and were generally detected more frequently than the HIV-1 subtype F in most studies, except in Pernambuco, where subtype F was endemic. High frequencies of HIV-1 recombinant BF, ranging from 3.3% to 42.5%, were detected in Bahia. Recombinants BC and AG were identified less frequently than BF. Rare genotypes, such as HIV-1 subtype D and recombinants BD, AB, and BCF, were also identified and their frequencies determined (Table 1).
| Authors | Yeara | Statesb | Sequencing dates | HIV genetic region analyzed | Nc | HIV-1 subtypes, N (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | F | C | D | BF | DF | AG | BC | BCF | AB | BD | KB | CF | URFs | NI | ||||||
| Abreu et al | 2017 | BA | 2008-2012 | Pol (PR + partial RT)d | 101 | 68 (67.4) | 8 (7.9) | 2 (1.9) | 18 (17.8) | 1 (0.99) | 1 (0.99) | 1(0.99) | 2 (1.9) | |||||||
| Alencar et al11 | 2013 | SP, PE, MG, RJe | 2009 | Pol (PR + partial RT) | 109 | 80 (73) | 26 (24) | 2 (2) | 1 (1) | |||||||||||
| Amaral et al | 2017 | BA | 2012 | Gag, pol, env | 263 | 179 (68.1) | 23 (8.7) | 5 (1.9) | 55 (20.9) | 1 (0.4) | ||||||||||
| Araújo et al | 2010 | BA | 2006 | Gag, env | 61 | 56 (91.8) | 3 (4.9) | 2 (3.3) | ||||||||||||
| Arruda et al | 2010 | CE | 2008-2009 | Pol (PR + partial RT) | 74 | 63 (85.1) | 6 (8.1) | 4 (5.4) | 1 (1.4) | |||||||||||
| Arruda et al | 2018 | Brazil | 2013-2015 | Pol (PR + partial RT) | 265 | 204 (77) | 28 (10.8) | 11 (4.2) | 01 (0.4) | 05 (1.9) | 16f (6.0) | |||||||||
| Brennan et al12 | 2007 | BA, CE, GOe | 2001-2003 | Gag, pol, env | 78 | 59 (75.6) | 3 (3.8) | 16 (20.5) | ||||||||||||
| Brindeiro et al | 2003 | RS, PR, RJ, MS, SP, BA, CEe | 2001 | Pol (PR + partial RT) | 42 | 36 (86.3) | 2 (4.5) | 4 (9.0) | ||||||||||||
| Cavalcanti et al | 2007 | PI, CE, RN, PB, PE, AL | 2002-2004 | Pol (PR + partial RT) | 502 | 413 (82.4) | 59 (11.8) | 5 (1) | 1 (0.2) | 24 (4.6) | ||||||||||
| Cavalcanti et al13 | 2012A | PE | 2007-2009 | Pol (PR + partial RT) | 169 | 59 (54.6) | 43 (39.8) | 3 (2.8) | 3 (2.8) | |||||||||||
| Cavalcanti et al14 | 2012B | PE | 2007-2009 | Pol (PR + partial RT) | 130 | 74 (56.9) | 49 (37.7) | 4 (3.1) | 3 (2.3) | |||||||||||
| De Moraes Soares et al15 | 2014 | AM, BA, DF, RJ, RSe | 2009-2010 | Pol (PR + partial RT) | 47 | 33 (70.2) | 7 (14.9) | 2 (4.3) | 5 (10.6) | |||||||||||
| Delatorre et al | 2017 | AL, BA, CE, PI | 2014-2015 | Pol (PR + partial RT) | 140 | 101 (72) | 9 (6) | 7 (5) | 1 (1) | 15 (11) | 1 (1) | 5 (4) | 1 (1) | |||||||
| Dourado et al16 | 2007 | BA | 1998-2000 | Gag, pol, env | 13 | 11 (84.6) | 2 (15.4) | |||||||||||||
| Filho et al17 | 2017 | BA | 2008-2014 | Pol (PR + partial RT) | 1300 | 1020 (78.5) | 87 (6.7) | 85 (6.5) | 108 (8.2) | |||||||||||
| Gadelha et al18 | 2003 | CE | 2000 | Gag, env | 149 | 121 (93.8) | 4 (3.1) | 4 (3.1) | ||||||||||||
| Guimarães et al | 2015 | PE, BA, DF, MS, MG, RJ, PR, SCe | 2009 | Pol (PR + partial RT), env | 33 | 18 (54.5) | 1 (3) | 14 (42.5) | ||||||||||||
| De Medeiros et al19 | 2006 | PE | 2002-2003 | Pol (PR + partial RT) | 84 | 61 (72.6) | 19 (22.6) | 1 (1.2) | 3 (3.6) | |||||||||||
| Monteiro et al | 2009 | BA | 2002 | Gag, env | 175 | 143 (81.7) | 01 (0.6) | 30 (17.1) | 01 (0.6) | |||||||||||
| Monteiro-Cunha et al20 | 2011 | BA | 2007 | Pol (PR + partial RT) | 58 | 39 (67.2) | 4 (6.9) | 1 (1.7) | 14 (24.2) | |||||||||||
| Moura et al | 2015A | MA, PI | 2012 | Pol (PR + partial RT) | 106 | 86 (81.1) | 2 (1.9) | 3 (2.8) | 13 (12.3) | 02 (1.9) | ||||||||||
| Moura et al21 | 2015B | PI | 2011-2012 | Pol (PR + partial RT) | 89 | 77 (86.6) | 1 (1.1) | 1 (1.1) | 04 (4.5) | 06 (6.7) | ||||||||||
| Oliveira et al22 | 2018 | PE | 2006-2016 | Pol (PR + partial RT) | 171 | 139 (81.4) | 31 (18.2) | 1 (0.4) | ||||||||||||
| Pessôa et al23 | 2014A | SP, MG, PE, RJe | 2007-2011 | Near full-length genomeg | 11 | 9 (81.8) | 1 (9.1) | 1 (9.1) | ||||||||||||
| Santos et al | 2009 | BA | 2008-2009 | Pol (PR + partial RT) | 52 | 33 (63.6) | 4 (7.6) | 1 (1.9) | 14 (26.9) | |||||||||||
| Santos et al | 2011 | BA | 2006 | Pol, env | 57 | 45 (78.9) | 3 (5.3) | 9 (15.8) | ||||||||||||
| Vaz et al24 | 2015 | BA | 2012-2013 | Gag, pol, env | 19 | 34 (82.1) | 7 (17.9) | |||||||||||||
- NI = not informed.
- 20-33 = numbers equivalent to reference topic citation.
- a Year of publication of the study.
- b States of Northeast Brazil: AL = Alagoas, BA = Bahia, CE = Ceará, MA = Maranhão, PB = Paraíba, PE = Pernambuco, PI = Piauí, RN = Rio Grande do Norte, SE = Sergipe.
- c N, the sample size refers to the number of samples sequenced as reported by the articles. That is, samples not sequenced are excluded, allowing the real frequency of HIV-1 subtypes to be obtained.
- d PR = protease; RT = reverse transcriptase genes.
- e Some articles contain data from other regions of Brazil. However, only data from the Northeast region had been demonstrated.
- f The type of URF detected is not specified.
- g Near full-length genome = five fragments of HIV-1 genome with approximately 9000 bp.
HIV-1 subtype distribution data from Northeast Brazil were grouped by states; Bahia and Pernambuco were found to have the largest number of sequenced samples (Figure 2). HIV-1 B was the main observed subtype in all states in the region. However, certain local characteristics were noted regarding HIV molecular epidemiology. High frequencies of BF recombinants (>11%) and HIV-1 F (25%) were detected in Bahia and Pernambuco, respectively. Additionally, BC recombinants were found to be greater than 3% and 5% in Alagoas and Piauí, respectively. No study of HIV-1 molecular epidemiology in Sergipe was identified and the only study to report data from Maranhão25 did not specify the number of HIV-1 genotyped patients analyzed in each state (Table 2).
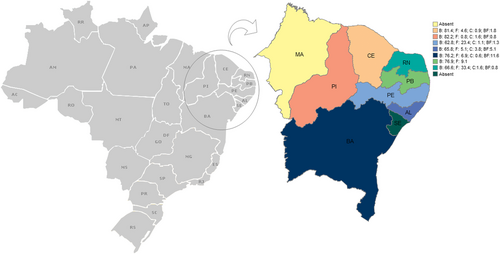
| States—Northeast Brazila | Nb | Viral subtype and recombinants (HIV) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B, n (%) | F, n (%) | C, n (%) | BF, n (%) | DF, n (%) | AG, n (%) | BC, n (%) | AB, n (%) | BD, n (%) | KB, n (%) | BCF, n (%) | ND, n (%) | ||
| Alagoas | 79 | 52 (65.8) | 4 (5.1) | 3 (3.8) | 4 (5.1) | ⋯ | ⋯ | 3 (3.8) | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| Bahia | 2284 | 1740 (76.2) | 158 (6.9) | 13 (0.6) | 264 (11.6) | 2 (<0.1) | 01(<0.1) | 1 (<0.1) | 1 (<0.1) | 1 (<0.1) | 2 (<0.1) | ⋯ | 108 (4.7) |
| Ceará | 560 | 456 (81.4) | 26 (4.6) | 5 (0.9) | 10 (1.8) | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| Paraíba | 13 | 10 (76.9) | 1 (9.1) | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| Pernambuco | 931 | 585 (62.8) | 218 (23.4) | 10 (1.1) | 12 (1.3) | ⋯ | ⋯ | 01 (0.1) | ⋯ | ⋯ | ⋯ | 1 (0.1) | ⋯ |
| Piauí | 124 | 102 (82.2) | 01 (0.8) | 02 (1.6) | 01 (0.8) | ⋯ | ⋯ | 07 (5.6) | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| Rio Grande do Norte | 3 | 2 (66.6) | 1 (33.4) | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
- a No HIV-1 samples were determined for the states of Sergipe and Maranhão.
- b Total number of sequenced samples may not be equal to the sum of the described subtypes and recombinants numbers, as some studies lacked information about subtype distribution from the number of sequenced samples from states in the Northeast Brazil. In addition, Arruda et al4 only described HIV-1 genetic diversity for the Brazilian regions in general.
3.2 Estimated frequency of major HIV-1 subtypes and recombinants from Northeast Brazil (meta-analysis)
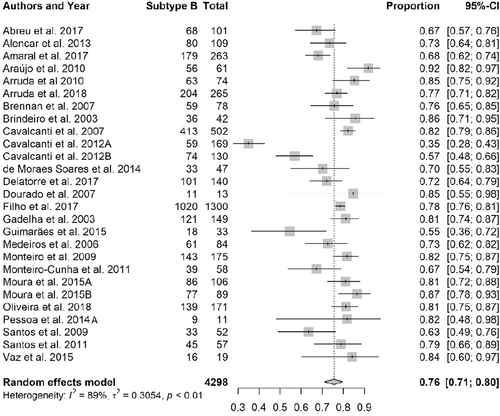
Estimated frequency of subtype B in the 27 selected studies ranged from 35% (95% CI: 28-43%) to 92% (95% CI: 82-97%), the mean being 76% (95% CI: 71-80%). There was a great heterogeneity in the data, depending mainly on the state where the HIV-1 sequences were obtained; however, most studies presented frequencies higher than the mean obtained by the meta-analysis (Figure 3).
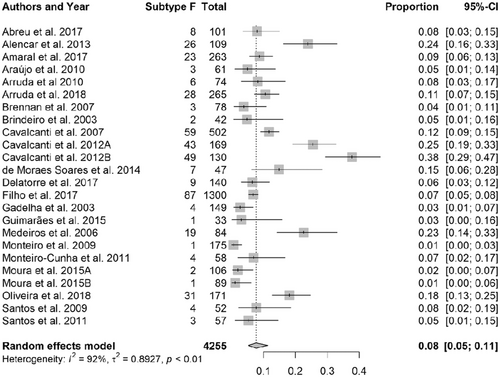
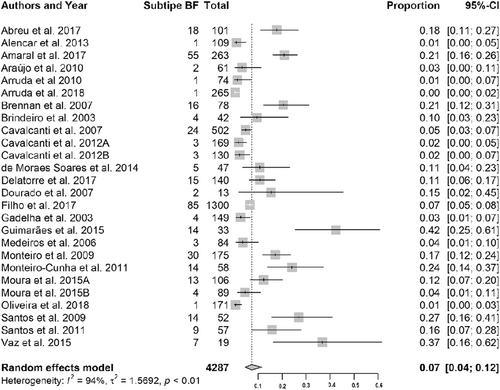
Subtype F frequency ranged from 1% (95% CI: 0-3%) to 38% (95% CI: 29-47%), the mean being 8% (95% CI: 5-11%). The high heterogeneity in the data presented in the meta-analysis was due to data from the articles reporting sequences obtained in Pernambuco (Table 1 and Figure 4).
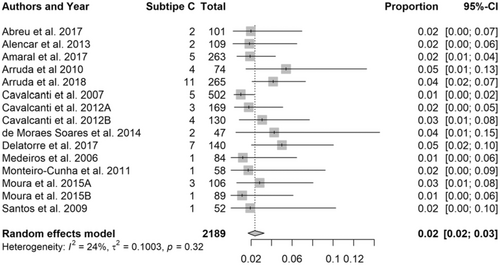
Subtype C had the lowest frequencies, ranging from 1% (95% CI: 0-2%) to 5% (95% CI: 1-13%), the mean being 2% (95% CI: 2-3%) (Figure 5). The BF recombinant showed high dispersion in Northeast Brazil, being cited in practically all studies, except for only one (Figure 6). However, they presented great heterogeneity in the meta-analysis and frequency distribution data, with high frequencies in Bahia.
4 DISCUSSION
We present the first systematic review and meta-analysis of HIV-1 subtype distribution in Northeast Brazil from 27 studies published between January 1999 and August 2019. Subtype B was predominant in all analyzed studies, followed by subtypes F and C, respectively. Frequencies of different HIV-1 subtypes were similar to those previously described for most regions of Brazil. Arruda et al.4 described the subtype B frequency to be 77% in the Northeast, followed by subtype F with a frequency of 10.6%. The largest number of articles about HIV-1 molecular epidemiology in Northeast Brazil were reported from Bahia, with 2284 samples being analyzed. Of the 15 publications found in the databases, a large variation between subtypes was observed. The subtype B frequency ranged from 63.6%26 to 91.8%.27 It is important to note that in addition to the high frequency of HIV-1 BF recombinants, other recombinants were found in the state of Bahia: DF,28, 29 KB, AG, AB, and DB,30 two patients having DF and KB recombinants and one patient each with AG, AB, and DB recombinants.
Pernambuco was the second most studied state in Northeast Brazil, with eight articles being published reporting a subtype B frequency range of 54.6%14 to 81.8%,31 followed by subtype F (frequency range of 18.231-39.8%14). According to our analysis, Pernambuco has a HIV-1 subtype B frequency (62.8%).31 In addition to the high frequency of HIV-1 subtype F, viral recombinants such as BF, BC, and BCF have also been reported.31 The first two CRFs identified in the Northeast, CRFs 70 and 71_BF,31 were also detected in this state. Therefore, we noted that Pernambuco and Bahia had the largest number of samples and showed great HIV-1 diversity, including a large circulation of viral recombinants. However, we cannot claim whether this high frequency of recombinants in the two largest states in the region is due to multiple entries of pure subtypes followed by potential intersubtype recombination and their dispersion, due to founder and dispersive effect of these recombinants or caused by other factors. In Alagoas, the highlights are the circulation of HIV-1 C and BC (3.8% for both), accompanied by the lower HIV-1 subtype B frequency than that in the Northeast (65.8%). In contrast, Ceará and Piauí had the highest frequencies of HIV-1 subtype B for the region (>80%).
Despite the large number of articles on HIV-1 subtype distribution in Northeast Brazil, this study has some limitations, such as the bias for HIV-1 subtyping in several articles, as the different methodologies employed for subtyping may have interfered with the accuracy of determining the true viral subtype. Another important aspect is the extent of the viral genomic region analyzed (whole genome or a specific region), as analysis of the complete or near-full genome is highly sensitive for detecting possible recombination. In addition, some regions of the viral genome function more effectively as hotspots for intersubtype recombination than others. Regarding the results of the HIV-1 subtype distribution in the different states of the region, it is important to highlight that information bias has been identified in certain articles as some of them do not report any information about viral subtype distribution individualized by each state, providing only general information about viral diversity in the region.
5 CONCLUSION
This study provides an updated integration of available data on the prevalence of HIV-1 subtypes in Northeast Brazil from 1999 to 2019, highlighting a large viral diversity. Molecular virology data are important to determine the dynamics of the HIV epidemic in the region and provide insights into the possibility of probable differences between the different subtypes and recombinants in response to antiretroviral therapy, transmission of resistance mutations, progression to disease, and viral spread. Thus, molecular epidemiological data are essential for strategies for prevention and control of the HIV/AIDS epidemic.




