Relevance of reviewing endpoint analysis for negative results on the Xpert Xpress Flu/RSV
Abstract
After the implementation of the Xpert Xpress Flu/respiratory syncytial virus (RSV) assay for rapid respiratory molecular testing, we investigated the significance of reported endpoint values for influenza A, influenza B, and RSV). This study prospectively analyzed nasopharyngeal swabs submitted to our virology laboratory in the 2018/19 influenza season. Initial testing was performed on the Xpress Flu/RSV assay. Samples were further tested on a laboratory-developed multiplex polymerase chain reaction (laboratory-developed multiplex respiratory test [LDT]) if the sample was reported as negative by the Xpress Flu/RSV but had an elevated endpoint value ≥5 for any respiratory virus target. There were 1040 negative results on the Xpress Flu/RSV; thirty-one had at least one endpoint value ≥5 [influenza A (25), influenza B (1), RSV (2), influenza A/RSV (1), and influenza A/B/RSV (2)]. Five samples (5/31, 16.1%) were positive on the LDT for influenza A or RSV. In contrast, the positivity rate on the LDT for negative Xpress Flu/RSV samples with endpoint values less than 5 was 0.35% (P < .0001). A threshold for endpoint values could not reliably be established to differentiate a potential influenza A positive result from a negative result on the LDT. Routine evaluation ofendpoint values should be a consideration for laboratories implementing Xpress Flu/RSV, in addition to supplementary respiratory virus testing for clinically relevant situations.
1 INTRODUCTION
Rapid molecular testing for respiratory viruses such as influenza A, influenza B, and respiratory syncytial virus (RSV) is being increasingly adopted in healthcare facilities. With a rapid turn-around-time and increased sensitivity compared to nonmolecular methods, these assays present an opportunity to improve patient care through optimization of antimicrobial treatment and placement of patients on appropriate infection prevention and control precautions.1, 2
At our institution, we implemented a tiered testing protocol for respiratory viruses in which a rapid polymerase chain reaction (PCR) test for influenza A, influenza B, and RSV is performed in the virology laboratory. Based on clinical criteria, samples may be selected for further testing on a laboratory-developed multiplex PCR panel run once every 24 hours which detects parainfluenza 1 to 3, adenovirus, and human metapneumovirus in addition to influenza A, influenza B, and RSV. Clinical criteria included any one of the following: admission to the intensive care unit; transplant recipient; human immunodeficiency viruses (HIV)-positive patient; cystic fibrosis patient; or a patient meeting antimicrobial stewardship criteria (negative bacterial cultures on current admission, absence of new lobar consolidation or pneumonia as reported by radiologist on chest imaging, AND antibiotics prescribed for possible pneumonia).
Our virology laboratory had limited experience with the new rapid assay (Xpert Xpress Flu/RSV [Cepheid, Sunnyvale, CA]) which reports cycle threshold (Ct) values as well as reaction endpoint values for each target on the assay (two targets for influenza A [A1 and A2], one target for influenza B, and one target for RSV). There are no formal definitions of the “endpoint,” but package insert materials suggest it is a measure of the total amount of fluorescence detected at the end of the final nucleic acid amplification cycle.3 To determine the significance of these values, we implemented a precautionary protocol: the laboratory-developed multiplex PCR test was performed on samples reported as negative by the Xpress Flu/RSV but for which a detectable endpoint value was provided for influenza A, influenza B or RSV.
2 METHODS
During 2018 to 2019 respiratory virus season, we prospectively analyzed all nasopharyngeal swabs submitted to our virology laboratory, which provides testing for one tertiary care academic hospital, one community hospital, and five long-term care facilities. Initial testing for respiratory viruses was performed on the Xpress Flu/RSV. All patients with negative results were reviewed for clinical criteria as previously described; if criteria were met, the sample was further tested on a laboratory-developed multiplex PCR (laboratory-developed multiplex respiratory test [LDT]). In addition, the endpoint values for all samples testing negative on the Xpress Flu/RSV assay were reviewed. If any endpoint value was ≥5 for influenza A, influenza B or RSV, the sample was further tested on the LDT. The endpoint cut-off of ≥5 was selected based on the observation that the vast majority of negative results on the Xpress Flu/RSV have reported endpoint values of 0 to 1. The LDT has been previously described,4 and is based on the Centers for Disease Control and Prevention protocol for influenza A and B. It is composed of four duplex/triplex reactions (influenza A with the internal control, influenza B with parainfluenza 1, RSV with adenovirus and human metapneumovirus, and parainfluenza 2 with parainfluenza 3), and, therefore, all respiratory virus targets were tested in these cases. The assay has a threshold Ct value of ≤40. Discordant results between the Xpress Flu/RSV and the LDT were clinically reviewed by a Medical Microbiologist before reporting.
3 RESULTS
A total of 1040 negative test results on the Xpress Flu/RSV were reported. Thirty-one samples were reported as negative on the Xpress Flu/RSV, but had at least one endpoint value of ≥5. Of these samples, endpoint signals were identified in influenza A (25), influenza B (1), RSV (2), influenza A/RSV (1) and influenza A/B/RSV (2).
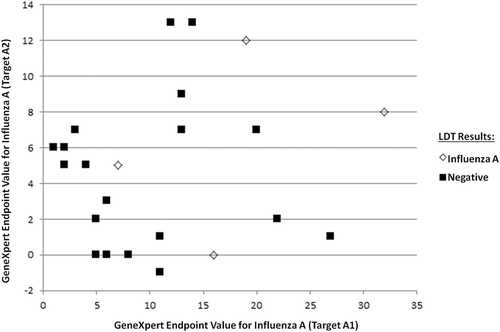
For the samples with an endpoint value ≥5 for either influenza A target on Xpress Flu/RSV (n = 28), the range of endpoint values were quite variable (5-32). These values could not reliably be used to differentiate a potential influenza A positive result from a negative result on the LDT, although no samples with an influenza A1 endpoint value greater than 27 in this study tested negative on the LDT (Figure 1). Regarding influenza B, only three samples were identified (endpoint values: 5, 5, 6) and subsequently tested negative on the LDT. There were five samples with an RSV endpoint (range: 5-24), and only one sample (with an endpoint value of 9) was confirmed as RSV positive by the LDT (Table 1).
| Sample | Xpress endpoint | Xpress endpointa value | LDT result | LDT Ct |
|---|---|---|---|---|
| 1 | Influenza A | 16, 0 | Influenza A | 40 |
| 2 | Influenza A | 19, 12 | Influenza A | 37.8 |
| 3 | Influenza A | 7, 5 | Influenza A | 40 |
| 4 | RSV | 9 | RSV | 40 |
| 5 | Influenza A | 32, 8 | Influenza A | 40 |
- Abbreviations: LDT, laboratory-developed multiplex respiratory test; RSV, respiratory syncytial virus.
- a Endpoints reported on the Xpress Flu/RSV for influenza A targets (Flu A1, Flu A2) and the RSV target (RSV).
Five samples (5/31, 16.1%) were positive on the LDT for influenza A or RSV, with their endpoint values and LDT Ct values listed in Table 1. One other sample with influenza A endpoint values of 0 and 6 subsequently tested positive for parainfluenza 3 on the LDT.
In contrast, 288 samples which tested negative on the Xpress Flu/RSV with endpoint values less than 5 were selected for further testing on the LDT as per the laboratory's tiered testing protocol. One sample (1/288, 0.35%) initially had a positive influenza A result on the LDT with a Ct value of 40. This result was not detected on repeat LDT testing and was determined to be clinically insignificant. None of the other samples had influenza A, influenza B or RSV detected on the LDT. The overall positivity rate (0.35%) in this group of samples compared to the positivity rate (16.1%) of samples with endpoint values ≥5 was significant (P < .0001; Fisher's exact test; QuickCalcs GraphPad software).
Last, samples with elevated endpoint values requiring supplemental testing on the LDT had an average turn-around-time of 24 hours and 12 minutes, compared to samples requiring only the rapid assay which had an average turn-around-time of 8 hours and 16 minutes.
4 DISCUSSION
Our laboratory did not initially require supplementary testing for detectable endpoint values when the Xpress Flu/RSV was first implemented as the package insert did not have specific instructions for interpreting such information off of the Xpress report.3 However, as the laboratory identified more samples with these values, follow-up testing was implemented as a quality control initiative to better understand such values. Review of endpoint analysis in this study identified 5/31 samples (16.1%) which initially were reported negative by Xpress Flu/RSV but had a detectable respiratory virus on the LDT, including four samples which were positive for influenza A. Although the clinical significance of low-level positive (Ct value ≥37) influenza A, influenza B, or RSV results is unclear, the detection of these respiratory pathogens could be significant depending on the clinical setting. Our virology laboratory serves patient populations from a broad range of clinical services, including high-risk units such as critical care and solid organ transplant units/clinics. As such, we believed these findings combined with the clinician's assessment would be relevant for consideration of optimal antimicrobial treatment. Of the five patients who had tested negative on the Xpress Flu/RSV but positive for a respiratory virus on the LDT, all required admission to hospital. Two were immunocompromised (untreated HIV; use of a biologic agent) and one had a predisposing pulmonary condition (chronic obstructive pulmonary disease). Oseltamivir was prescribed in all cases where the detection of influenza A was reported. Of interest, at least one of the patients presented to hospital after experiencing respiratory symptoms for over 1 week's duration. It has been demonstrated that Ct values increase as time from respiratory illness onset to time of testing is prolonged.5 Although individuals may be asymptomatic carriers of a respiratory virus, influenza A is more likely to be associated with symptomatic disease and is detectable for shorter periods of time compared to other respiratory viruses.6
Identification of influenza A, including those samples with a low level of positivity, can be of significant value to infection prevention and control and public health. Isolating patients with contact and droplet precautions is necessary to prevent transmission within a healthcare facility, and for prevention of potential outbreaks.7 Although the risk of secondary infection is reduced if the source patient tests “low positive” for influenza (Ct value >30) compared to source patients who test “high positive” for influenza (Ct value ≤30), the transmission risk is not negligible and secondary cases have been documented.5
Furthermore, the rapid assay significantly improved turn-around-time in comparison to the LDT. For samples with elevated endpoint values requiring supplemental testing on the LDT, the increased turn-around-time was acceptable as this matched the laboratory's baseline performance. Respiratory samples had historically been batched and testing on the LDT once every 24 hours. After implementation of the two-tiered testing protocol, clinical review of patients occurred daily and the LDT continued to be run once every 24 hours.
As a Clinical Laboratory Improvement Amendments of 1988 (CLIA)-waived test, there is the capability to perform Xpress Flu/RSV testing outside of the laboratory environment, though the test characteristics are predominantly derived from laboratory-based studies.8 Our study is limited by the fact it was performed in a hospital-based clinical virology laboratory with molecular expertise, and may not be as relevant in other settings. However, this study also highlights the importance of utilizing these CLIA-waived tests in molecular laboratories to develop experience in the interpretation of the results, as well as investigate discordant results and manage quality control/assurance.
Rapid molecular tests such as the Xpress Flu/RSV have demonstrated high sensitivity, with the reported sensitivities more than 95%compared to either laboratory-developed assays or commercial assays.9-11 Nevertheless, discordant results that have been previously described in the literature may be of significance for laboratories serving complex patient populations including those who are immunocompromised, post-transplant, or in critical care. Although the current investigation of samples testing negative on the Xpress Flu/RSV but with a detectable endpoint value could not quantitatively and definitively predict which samples have low levels of detectable respiratory virus on alternate platforms, the frequency of detection of respiratory viruses on the LDT was significantly higher in the group with endpoint values ≥5 compared to those where endpoint values were less than 5.
5 CONCLUSION
Evaluation of endpoint values should be a consideration for laboratories implementing Xpress Flu/RSV to avoid reporting potential false negative results. If significant endpoint values are identified, laboratories should consider confirmatory or supplementary respiratory virus testing for clinically relevant situations.
ACKNOWLEDGMENT
We would like to thank the staff in the St Paul's Hospital Virology Laboratory for their commitment to quality testing.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
NM: conceptualization, methodology, data curation, writing—original draft. GR: methodology, writing—review and editing. TL and LK: investigation. MGR: conceptualization, writing—review and editing. CFL: conceptualization, methodology, data curation, visualization, writing—original draft.




