Occult HCV and occult HBV coinfection in Iranian human immunodeficiency virus-infected individuals
Abstract
The presence of hepatitis C virus (HCV) genome in liver biopsy or peripheral blood mononuclear cell (PBMC) specimens in the absence of detectable HCV-RNA in plasma of the people with or without anti-HCV antibodies has defined as occult HCV infection (OCI), whereas occult hepatitis B virus infection (OBI) is detection of hepatitis B virus (HBV) genome in the absence of traceable hepatitis B surface antigen in the plasma samples of patients. The purpose of this study is to determine the presence of OBI and OCI in human immunodeficiency virus (HIV)-infected individuals. In this cross-sectional research, 190 Iranian HIV-infected individuals were enrolled from September 2015 to February 2019. All participants were tested regarding various serological markers for HCV and HBV infections. Viral RNA and DNA were extracted from plasma and PBMC specimens, and the presence of HCV-RNA in plasma and PBMC samples was tested using reverse transcriptase-nested polymerase chain reaction (PCR), HBV viral load was determined in plasma samples using COBAS TaqMan 48 Kit, and also the presence of the HBV-DNA in PBMC samples was tested by real-time PCR. In this study, the prevalence of OBI and OCI in HIV-infected individuals was 3.1% and 11.4%, respectively. The genotypes of HCV in the patients with OCI were as follows: 57.1% were infected with subtype 3a, 35.7% were infected with subtype 1a, and 7.1% was infected with subtype 1b. It is noteworthy that in this study, two patients (1.1%) had OCI/OBI coinfections. The present study revealed that 1.1% of Iranian HIV-infected individuals had OBI and OCI at the same time. Therefore, it seems that designing prospective surveys to determine the presence of this coinfection in HIV-infected individuals is informative.
1 INTRODUCTION
Hepatitis B and C are propounded as major public health issues worldwide. Morbidity and mortality due to viral hepatitis have been remarkable in recent decades. Viral hepatitis can result in several sign and symptoms (from mild and chronic disease to acute and severe one, which leads to cirrhosis and hepatocellular carcinoma [HCC]).1 Viral hepatitis and human immunodeficiency virus (HIV) infection as blood-borne diseases have similar and common routes of transmission, as a result, coinfection of viral hepatitis and HIV infection is inevitable. According to the World Health Organization reports, 37 million people are suffering from HIV infection, hepatitis C virus (HCV) infection has a global impact in terms of morbidity and mortality with more than 70 million individuals are affected by viral hepatitis, and the coinfection of these viral infections is dramatically leading to severe liver disease and death in HIV-positive individuals, which is inversely related to the CD4 T-cell counts.2-4 On the other hand, the complications of interferential treatment strategies can affect the quality of life and treatment process.5
Occult hepatitis B virus infection (OBI) is a clinical class of hepatitis B virus (HBV) infection characterized by the presence of HBV-DNA in the liver or blood and also the absence of hepatitis B surface antigen (HBsAg) in plasma or serum. There are two clinical types of OBI; seropositive OBI, in which hepatitis B core antibody (Anti-HBcAb) and/or hepatitis B surface antibody (Anti-HBs Ab) is positive and seronegative OBI, in which these two antibodies are absent.6 The gold standard for OBI diagnosis is detection of HBV-DNA from liver tissue with sensitive techniques, such as nested polymerase chain reaction (PCR) and real-time PCR.7
The prevalence of OBI is varied worldwide depending on many indices, such as HBV-DNA detection techniques, like nested PCR or real-time PCR, sample size, and detection from liver or blood samples, so the prevalence rate of OBI is ranged from 1% up to 87% and is varied in different regions. In the Eastern Asia, the prevalence of OBI is greater than other regions. The prevalence of OBI in patients with HCV infection is 15% to 33% and also a prevalence of 10% to 45% has reported for HIV and OBI coinfection. The prevalence of this infection is significantly higher in people with HCC (up to 62%). It is worthy noted that OBI in HIV-infected individuals can result in the faster progression of liver fibrosis, cirrhosis, and HCC and sometimes may cause fulminant hepatitis.3, 6, 8
Occult HCV infection (OCI) is characterized by no detectable anti-HCV antibodies (Abs) in patient's plasma or serum, but detectable HCV-RNA in liver biopsy specimens as a gold standard test, and in 70% of cases in peripheral blood mononuclear cell (PBMC) samples.9 In OCI, HCV-RNA negative strands as an intermediary of PBMCs act as extrahepatic source of HCV replication.10 OCI have three clinical patterns, in which the absence of HCV-RNA in serum or plasma is common; (a) the absence of anti-HCV Ab in plasma sample and evaluated liver enzymes, (b) the presence of anti-HCV Ab in spontaneous patients, and (c) the presence of anti-HCV Ab in patient's serum or plasma in treatment-induced HCV clearance.11
In contrast to OBI, the prevalence of OCI is varied worldwide, but it is not depended on alternation indices. The prevalence of OCI in HBV infection is reported 28% to 40%. Although OCI/HBV coinfection can inhibit HBV replication, however, in these patients, the level of liver enzymes was reported higher than patients without OCI, and liver fibrosis progress is higher in patients with OCI.12 Few studies are available on OCI in HIV-infected individuals. In a study in Georgia, the high prevalence of OCI (31%) has reported in HIV/HBV-infected individuals.13
Intellectual strategies of occultation in HBV and HCV, accompanied by HIV infection, have challenged the public and global health and diagnosis and treatment of these patients are associated with several complications. According to the limited available data on this issue, this study was performed to determine the prevalence of occult HCV and occult HBV infections in HIV-positive people.
2 MATERIALS AND METHODS
2.1 Study population
This cross-sectional research was conducted on 190 recently diagnosed antiretroviral treatment-naïve Iranian HIV-infected individuals (HIV Ag/Ab and HIV-RNA positive) who were referred to hospitals affiliated to Iran University of Medical Sciences, Tehran, Iran, between September 2015 and February 2019. All of the participants were cytomegalovirus (CMV) immunoglobulin M and CMV-DNA negative, and also all known autoimmunity (negativity for antimitochondrial and antinuclear antibodies, etc), genetic disorders, and drug toxicity were negative.
2.2 Collection of the specimens
Six microliters of peripheral blood was taken from each patient into a sterile ethylenediaminetetraacetic acid-containing vacutainer tubes. After separation of the plasma by centrifugation (3000 rpm for 5 minutes), plasma was stored at –70℃ for the viral RNA and DNA extraction. The PBMCs of the samples were isolated from the remainder of the blood using a standard method by Ficoll Hypaque density gradient (Lympholyte HTM; Cedarlane, Hornby, Canada) centrifugation, and then separated PBMCs were washed (three times) with phosphate-buffered saline (pH 7.3 ± 0.1). The pellet of isolated PBMCs was resuspended in 400 µL RNALater solution (Ambion, Inc, Austin, TX), and kept at –20℃ for the viral RNA and DNA isolation.
Plasma and PBMC samples from 10 subjects who were infected with HCV, and 10 individuals who were infected with HBV were used as positive controls of HCV and HBV infection, respectively, and also plasma and PBMC specimens from 10 blood donors were used as negative controls.
2.3 Serologic tests by enzyme immunoassay
Anti-HCV ABs and HBV serological markers such as, HBsAg, hepatitis e antigen (HBeAg), hepatitis B e antibody (HBeAb), and HBcAb were analyzed by the commercial enzyme immunoassay kits (DIA.PRO, Milano, Italy), according to the manufacturer's procedure.
2.4 RNA extraction of the samples
To detect HCV-RNA in the plasma and PBMC samples of studied subjects, the viral RNA was isolated from 200 μL of plasma and from approximately 3-5 × 106 PBMC specimens by the High Pure Viral Nucleic Acid Kit (Roche Diagnostics GmbH, Mannheim, Germany), according to the manufacturer's protocol, and then the quality and quantity of the isolated RNA was evaluated by the NanoDrop spectrophotometer (Thermo Fisher Scientific, Wilmington).
2.5 Detection of HCV RNA using reverse transcriptase-nested PCR and HCV genotyping with restriction fragment length polymorphism method
The genomic HCV-RNA was detected in plasma and PBMC specimens by reverse transcriptase (RT)-nested PCR method using special primers for the 5′-untranslated region of HCV. Complementary DNA (cDNA) was synthesized using 0.5 mg of the extracted RNA, and the HCV-RNA was amplified by two set of primers as described previously in detail.14 The PCR products of patient's specimens, positive control samples, negative control specimens, and DNA size marker (100 bp) were electrophoresed on a 2.0% gel agarose and stained with safe stain and then visualized by a UV transilluminator.
For determining the genotypes of HCV in positive specimens, restriction fragment length polymorphism (RFLP) method was performed, as previously described in detail.14 To determine the type of HCV genotype, the PCR products were digested with special restriction enzymes (ScrFI, HinfI, MvaI, and BstUI) (Fermentas GmbH, St. Leon-Rot, Germany), and then the digested PCR products of HCV positive samples, undigested (173 bp) PCR products, positive and negative controls, and 50 bp DNA size marker were electrophoresed on a 3.0% gel agarose, stained by safe stain, and then visualized using a UV transilluminator. The genotypes and subtypes of HCV (1a, 1b, 2a, 2b, 3a, 3b, 4, 5, and 6) could be determined based on the fragmentation pattern of the digested PCR products.14
2.6 Amplification of the nonstructural protein 5B region of HCV and sequencing
After cDNA synthesis (as above), the HCV-RNA was amplified using two set of specific primers for the nonstructural protein 5B (NS5B) region by RT-nested PCR, as described previously in detail, to confirm the results obtained from the detection and genotyping of the HCV.15 The PCR products (629 bp) of the NS5B gene were purified by a QIAquick Extraction Kit (QIAGEN GmbH, Hilden, Germany) and then the purified PCR products were sequenced bidirectionally using an ABI 3730XL sequencer with dye termination method. Also, the nucleotide sequences obtained from the NS5B region amplification were submitted in the GenBank database (accession numbers: MN729336 to MN729349).
2.7 Analysis of NS5B sequences
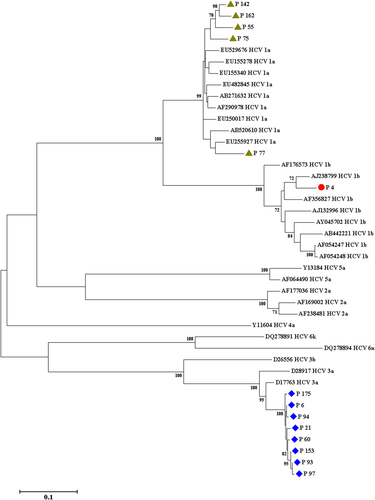
The sequences of NS5B region that obtained from this survey were aligned with all various reference sequences (retrieved from GenBank) of HCV (retrieved from GenBank database), using MEGA software version 7. A phylogenetic tree was drawn using the neighbor-joining method (Figure 1), and the statistical significance of this phylogenetic tree was analyzed by the bootstrap method (1000 replicates).
2.8 Viral load test of HBV
HBV viral load in 500 μL of the studied participants’ plasma samples was determined by the COBAS TaqMan 48 kit (Roche Diagnostics, Hacienda Drive Pleasanton, CA) and High Pure DNA Extraction Kit, according to the manufacturer's protocol. This assay is a real-time PCR method that is based on dual-labeled hybridization probe which targets the precore and core regions of HBV, and detection limit of this kit (COBAS TaqMan 48; Roche) is nearly 6> to 1 × 108 IU/mL.
2.9 Real-time PCR of the PBMC specimens for detection of HBV-DNA
The viral DNA was extracted from a pellet of PBMC specimens (4-6 × 106 cells) by QIAamp DNA Mini Kit (QIAGEN GmBH), according to the manufacturer's procedure.
The real-time PCR assay for the determination of the presence of HBV-DNA in the PBMC samples of the studied patients was carried out using the Rotor-Gene Q (QIAGEN GmbH) instrument.16 A pair of primers and a probe for amplification of a conserved region of the HBV surface gene were used, and also the human β-globin gene was used as internal control of real time PCR, as previously described in detail.16, 17
2.10 Statistical analysis
Statistical analysis was conducted by SPSS version 20 software (SPSS Inc, Chicago, IL). The Kolmogorov-Smirnov test was used to determine the normality of the quantitative variables. The analysis of continuous variables was carried out using one-way analysis of variance and Kruskal-Wallis tests. Statistical differences among the two groups were evaluated using χ2 test and Fisher's exact test when appropriate. P < .05 was considered statistically significant.
3 RESULTS
A total of 190 HIV infected individuals (anti-HIV Abs and HIV-RNA positive) were enrolled in this cross-sectional research. The mean age of studied subjects was 36.5 ± 13.2 years (range 1-68 years). Of 190 participants evaluated, 120 (63.2%) were male. All the information about demographic, laboratory, and epidemiological parameters of the studied patients are summarized in Tables 1 and 2.
| Male | Female | Total | P value | |
|---|---|---|---|---|
| Parameters | ||||
| Number of patients | 120 (63.2%) | 70 (36.8%) | 190 (100%) | |
| Age, y ± SD | 36.1 ± 12.4 (2-68) | 35.0 ± 14.6 (1-61) | 36.5 ± 13.2 (1-68) | .638 Mann-Whitney U |
| Laboratory parameters | ||||
| Viral load, IU/mL (median) | 636.0 (57-1 902 280) | 2765.0 (68-130 877) | 664.0 (57-1 902 280) | .914 Mann-Whitney U |
| CD4 count | 514.5 ± 757.7 (22-4780) | 429.3 ± 224.9 (74-1140) | 483.7 ± 620.0 (22-4780) | .284 Mann-Whitney U |
| ALT, IU/L | 52.8 ± 22.7 (17-180) | 42.2 ± 16.4 (10-78) | 48.9 ± 21.2 (10-180) | <.001* Mann-Whitney U |
| AST, IU/L | 48.5 ± 20.9 (18-168) | 39.0 ± 15.8 (11-71) | 45.0 ± 19.7 (11-168) | <.001* Mann-Whitney U |
| HCV Ab | 75 (62.8%) | 10 (14.3%) | 85 (44.7%) | <.001* Fisher's exact test |
| HCV RNA in plasma | 41 (34.2%) | 6 (8.6%) | 47 (24.7%) | <.001* Fisher's exact test |
| HCV RNA in PBMC | 48 (40.0%) | 13 (18.6%) | 61 (32.1%) | .004* Fisher's exact test |
| HBsAg | 15 (12.5%) | 8 (11.4%) | 23 (12.1%) | 1.000 Fisher's exact test |
| HBcAb | 45 (37.5%) | 6 (8.6%) | 51 (26.8%) | <.001* Fisher's exact test |
| HBeAg | 3 (2.5%) | 0 (0.0%) | 3 (1.6%) | .298 Fisher's exact test |
| HBeAb | 7 (5.8%) | 1 (1.4%) | 8 (4.2%) | .262 Fisher's exact test |
| HBV DNA in plasma | 7 (5.8%) | 0 (0.0%) | 7 (3.7%) | .048* Fisher's exact test |
| HBV DNA in PBMC | 9 (7.5%) | 0 (0.0%) | 9 (4.7%) | .028* Fisher's exact test |
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBcAb, hepatitis B core antibody; HBeAb, hepatitis e antibody; HBeAg, hepatitis e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HCV Ab, hepatitis C virus antibody; PBMC, peripheral blood mononuclear cell.
- * Statistically significant.
| Male | Female | Total | P value | |
|---|---|---|---|---|
| Parameters | ||||
| Number of patients | 120 (63.2%) | 70 (36.8%) | 190 (100%) | |
| Age, y ± SD | 36.1 ± 12.4 (2-68) | 35.0 ± 14.6 (1-61) | 36.5 ± 13.2 (1-68) | .638 Mann-Whitney U |
| Epidemiological parameters | ||||
| Injection drug user (IDU) | 79 (65.8%) | 3 (4.3%) | 82 (43.2%) | <.001* Fisher's exact test |
| IDU sexual partner | 0 (0.0%) | 46 (65.7%) | 46 (24.2%) | <.001* Fisher's exact test |
| History of having unprotected sex | 57 (47.5%) | 19 (27.1%) | 76 (40.0%) | .006* Fisher's exact test |
| History of tattooing | 39 (32.5%) | 3 (4.3%) | 42 (22.1%) | <.001* Fisher's exact test |
| History of needle stick | 33 (27.5%) | 1 (1.4%) | 34 (17.9%) | <.001* Fisher's exact test |
| History of imprisonment | 69 (57.5%) | 0 (0.0%) | 69 (36.3%) | <.001* Fisher's exact test |
| History of transfusion | 12 (10.0%) | 2 (2.9%) | 14 (7.4%) | .087 Fisher's exact test |
| Mother to child infection | 10 (8.3%) | 6 (8.6%) | 16 (8.4%) | 1.000 Fisher's exact test |
| Marital status | ||||
| Single | 59 (49.2%) | 14 (20.0%) | 73 (38.4%) | <.001* χ2 test |
| Married | 49 (40.8%) | 38 (54.3%) | 87 (45.8%) | |
| Divorced | 12 (10.0%) | 6 (8.6%) | 18 (9.5%) | |
| Widow | 0 (0.0%) | 12 (17.1%) | 12 (6.3%) | |
| Level of education | ||||
| Under Diploma | 74 (61.7%) | 48 (68.6%) | 122 (64.2%) | .138 χ2 test |
| Diploma | 33 (27.5%) | 15 (21.4%) | 48 (25.3%) | |
| Bachelor | 7 (5.8%) | 7 (10.0%) | 14 (7.4%) | |
| Master and Doctorate | 6 (5.0%) | 0 (0.0%) | 6 (3.2%) | |
- * Statistically significant.
The male sex is a significant risk factor in HCV (P < .001) and HBV (P = .048) coinfection. An impressive association was seen between the sex of the studied subjects and alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels (P < .001), HCV Ab (P < .001), HCV-RNA in plasma samples (P < .001), HCV-RNA in PBMC specimens (P = .004) (Table 1), injection drug user (IDU) (P < .001), IDU sexual partner (P < .001), history of having unprotected sex (P = .006), history of tattooing (P < .001), history of needle stick (P < .001), and history of imprisonment (P < .001), and marital status (P < .001) (Table 2).
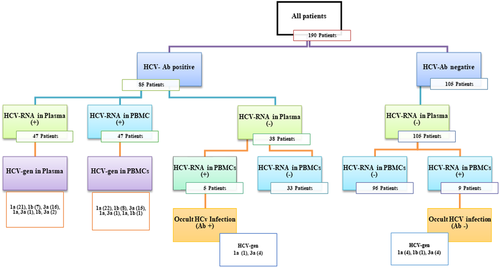
Eighty five (44.7%) cases of HIV-infected subjects were positive for anti-HCV Abs in plasma specimens; 47 (24.7%) had traceable HCV-RNA in the plasma specimens and 61 (32.1%) had detectable HCV-RNA in the PBMC samples (Table 1). Therefore, 47 studied participants with positive HCV-RNA test in their PBMC samples had traceable HCV-RNA in their plasma specimens and 14 cases were HCV-RNA negative in their plasma samples. These 14 (11.4%) studied subjects had OCI. Complete information about Iranian HIV-infected participants with OCI is summarized in Table 3. The HCV genotyping was carried out using RFLP assay for HCV positive samples. The results of HCV genotyping in plasma and PBMC samples were shown in Table 4. It is noteworthy that genotyping of HCV-RNA isolated from PBMC specimens of individuals with OCI was carried out using RFLP method, which demonstrated that eight (57.1%) subjects were infected with HCV subtype 3a, five (35.7%) subjects were infected with HCV subtype 1a, and one (7.1%) subject was infected with HCV subtype 1b (Table 3). Also, in the present study, there was an incompatibility between the genotypes of HCV in the plasma and PBMC specimens of seven (14.9%) individuals suffering from HIV/HCV coinfection (Table 4). Complete results of the serological and molecular virology evaluation of HCV infection in patients with HIV infection are showed in Figure 2.
| Parameters | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Age/sex | Viral load, IU/mL | CD4 Count | ALT, IU/L | AST, IU/L | HCV-Ab | HCV-RNA in Plasma | HCV-gen in plasma | HCV-RNA in PBMCs | HCV-gen in PBMCs | HBsAg | HBcAb | HBeAg | HBeAb | VL of HBV in plasma, IU/mL | HBV-DNA in PBMCs | Comments |
| 4 | 27/M | 126 581 | 408 | 71 | 67 | − | − | − | + | 1b | − | − | − | − | − | − | OCI |
| 6 | 64/M | 68 | 370 | 59 | 48 | + | − | − | + | 3a | − | − | + | − | − | − | OCI |
| 21 | 38/F | 2517 | 195 | 69 | 65 | + | − | − | + | 3a | − | − | − | − | − | − | OCI |
| 55 | 52/F | 115 | 80 | 68 | 56 | − | − | − | + | 1a | − | − | − | − | − | − | OCI |
| 60 | 37/M | 5182 | 620 | 59 | 54 | − | − | − | + | 3a | − | − | − | − | − | − | OCI |
| 75 | 39/M | 53 950 | 24 | 62 | 57 | − | − | − | + | 1a | − | − | − | − | − | − | OCI |
| 77 | 58/M | 3290 | 150 | 27 | 25 | + | − | − | + | 1a | − | + | − | − | − | − | OCI |
| 93 | 61/M | 57 620 | 473 | 74 | 67 | + | − | − | + | 3a | − | − | − | − | − | − | OCI |
| 97 | 37/M | 615 | 390 | 63 | 56 | − | − | − | + | 3a | + | − | − | − | − | − | OCI |
| 142 | 27/F | 129 422 | 490 | 75 | 71 | − | − | − | + | 1a | − | − | − | − | − | − | OCI |
| 153 | 52/F | 210 | 75 | 63 | 50 | − | − | − | + | 3a | − | − | − | − | − | − | OCI |
| 175 | 37/F | 3013 | 182 | 61 | 56 | + | − | − | + | 3a | − | − | − | − | − | − | OCI |
| 82 | 54/M | 79 | 264 | 77 | 74 | + | − | − | − | − | − | + | − | − | 187 | + | OBI |
| 102 | 40/M | 68 | 300 | 55 | 50 | + | − | − | − | − | − | + | − | + | 96 | + | OBI |
| 111 | 38/M | 1 157 880 | 79 | 32 | 37 | + | + | 1a | + | 1a | − | + | − | − | − | + | OBI |
| 184 | 36/M | 1 163 920 | 75 | 34 | 40 | + | + | 1a | + | 1a | − | + | − | − | − | + | OBI |
| 94 | 27/M | 620 | 643 | 39 | 36 | − | − | − | + | 3a | − | − | − | − | 154 | + | OBI/OCI |
| 162 | 27/M | 280 | 603 | 37 | 40 | − | − | − | + | 1a | − | + | − | − | 198 | + | OBI/OCI |
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; F, female; gen, genotype; HBcAb, hepatitis B core antibody; HBeAb, hepatitis e antibody; HBeAg, hepatitis e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; M, male; OBI, occult HBV infection; OCI, occult HCV infection; PBMC, peripheral blood mononuclear cell; VL, viral load.
| HCV genotypes | HCV genotypes in plasma | HCV genotypes in PBMCs | ||
|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | |
| 1a | 21 | 11.1 | 22 | 11.6 |
| 1b | 7 | 3.7 | 8 | 4.2 |
| 3a | 16 | 8.4 | 15 | 7.9 |
| 1a, 3a | 1 | 0.5 | 1 | 0.5 |
| 1a, 1b | 0 | 0 | 1 | 0.5 |
| 1b, 3a | 2 | 1.1 | 0 | 0 |
| Total | 47 | 24.7 | 47 | 24.7 |
- Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell.
There was a significant relationship between the sex of the studied participants and HBcAb (P < .001), HBV-DNA in plasma samples (P = .048), and HBV-DNA in PBMC specimens (P = .028) (Table 1). In this study, 167 (87.9%) participants were negative for HBsAg and 125 (65.8%) subjects were negative for all of the markers that show history of infection with HBV (such as HBV-DNA detection in plasma and PBMC specimens, HBsAg, HBcAb, HBeAg, and HBeAb). Eleven (5.8%) of the participants (4 men and 7 women) were positive for the HbsAg alone, therefore these individuals were HBV chronic nonactive carriers. On the other hand, 32 (16.8%) of the HIV-infected individuals were seropositive for HBcAb (6 women and 26 men), so they were isolated HBcAb positive.
The HBV-DNA was detected in the plasma specimens of seven (3.7%), and also in the PBMC samples of nine (4.7%) studied subjects (Table 1). It should be noted that, six cases of these participants had OBI. All of the patients with OBI were HBsAg negative, and genomic HBV-DNA was detectable in the plasma samples of four and in the PBMC specimens of six of them (Table 3). Interestingly, two of the patients with occult HBV infection were coinfected with HCV, and two others had OCI simultaneously (Table 3).
4 DISCUSSION
The last update of global epidemiology of viral hepatitis shows the high prevalence of viral hepatitis worldwide. Accordingly, the frequency of viral hepatitis in HIV-positive patients due to same transmission routes is high, as well.1 HIV infection and viral hepatitis are still the leading causes of mortality and morbidity in various developing countries, a lot of individuals directly exposed or people at high risk for HIV infection.18
Occult HBV infection is a form of HBV infection with the presence of HBV genome in the liver biopsy specimen, without or with detectable virus genome in plasma sample in HBeAg-negative individuals.6 On the other hand, a new unusual type of chronic hepatitis C, OCI, has been identified by Castillo et al,9 based on the presence of the HCV-RNA in the liver and/or PBMC specimens in the absence of anti-HCV Abs and the HCV-RNA in plasma samples.9 OBI and OCI have attracted more attention recently, since they appear to be related to the progressed liver fibrosis and cirrhosis.19 The current research aimed at determining the OBI and OCI in patients with HIV infection, and also the HBV/HIV, HCV/HIV, and HIV/HBV/HCV coinfections.
Among the 190 HIV-infected studied people, 105 subjects (55.3%) were negative for anti-HCV Abs and HCV-RNA in plasma samples, whereas the HCV-RNA was traceable in the PBMC specimens of nine (8.6%) subjects. Thus, these patients had OCI. It is noteworthy that HCV RNA was detectable in the PBMC specimens of 5 subjects (13.1%) of the 38 studied patients who were positive for anti-HCV Abs and negative for HCV-RNA in plasma specimens. These patients also had OCI (Figure 2). In general, in the present study, the prevalence of OCI in HIV-positive individuals (who were positive or negative for anti-HCV Abs and were negative for the HCV-RNA in plasma samples and positive for the presence of the HCV-RNA in PBMC specimens) was 11.4%.
Previous studies have shown that only in 70% of cases, this infection can be detected in the PBMCs of the individuals with OCI; therefore, observing the negative result for HCV RNA in a PBMC sample, does not exclude this infection (OCI) in the patient's liver biopsy specimen.9 So, it is likely that the prevalence of this infection in patients with HIV infection is higher than the percentage reported in this study.
OCI has been reported in various populations all over the world, for example, it has been diagnosed in hemodialysis patients in Germany (0.25%)20 and Thailand (18.2%)21; in people with glomerular nephropathies in Spain (39.0%)22; in people with cryptogenic liver disease in Iran (10.1%),10 Spain (57.0%),9 and Pakistan (74.0%)23; in those with HIV infection in Georgia (10.2%)13 and Iran (9.2%)24; in people with HBV infection (28%) in Italy25; among those with HCC in Italy (40.0%)23; in individuals with elevated ALT (32%) in Iran26; in patients with lymphoproliferative disorders (20%) in Egypt27 and Spain (13.3%)28; in individuals with various hematological disorders in Egypt (20%)29; in individuals with kidney transplantation (0.5%) in Germany20 and Egypt (3.7%)30; in general population in Italy (3.3%)31; and in volunteer blood donors in China (2.2%).32 Therefore, there are numerous reports of the presence of OCI in different groups of people worldwide. On the other hand, there are limited reports of the absences of OCI in various groups of patients,33-36 which should also be considered. Considering the results of recent studies and this study, more than 11% of HIV-positive individuals suffer from OCI, which may result in the accelerated liver fibrosis and liver failure due to immunosuppression.
There are approximately 2 278 400 HIV/HCV-coinfected patients in the world.3 In Iran, the prevalence of HIV/HCV coinfection has reported from 0.0% to 31.4%, with a median of 8.3%.37 In HIV-infected patients, imprisonment is one of the most important risk factors for transmission of viral hepatitis and HIV. Potential situations, such as sharing a needle, intravenous injection, men who have unprotected sex with men in prison and detention homes accelerate the risk of viral infections.38-41 In this study, 44.7% of the HIV-positive participants were infected with HCV, and also of 190 subjects, 14 subjects had OCI. The prevalence of OCI in HIV-positive subjects was 7.4%. RFLP genotyping of the HCV-positive cases in this study showed a different distribution pattern of HCV, as eight subjects (57.1%) were infected with HCV subtype 3a, five subjects (35.7%) were infected with HCV subtype 1a, and one subject (7.1%) was infected with HCV subtype 1b; however, in the general population in Iran, genotype 1a is predominant followed by genotype 3a.
Among 40 million people with HIV, up to 10% (2-4 million) are infected with HBV.42 It has known that the coinfection of these two viruses is due to their shared transmission routes. The prevalence of HBV/HIV coinfection is varied in different regions, for example, the East Asia and Sub-Saharan Africa have a high prevalence of HBV/HIV coinfection, whereas the North America and Western Europe are known as areas with a low prevalence of HBV/HIV coinfection.42
Hepatitis B infection was detected in up to 90% of the HIV-infected people (serological and molecular).43 It has reported that the prevalence of HBV/HIV coinfection is about 3.7% to 24.3% in Brazil, while two other studies have revealed a prevalence of 0.6% to 1.4% and 4.7% for HBV/HIV coinfection in France. Studies in Iran have shown that the prevalence of HBV and HBV/HIV coinfection are 30.9% and 1.9% in those who inject drugs, respectively.44 It is noteworthy that this coinfection is related to the chronic nature of HBV infection, and is also one of the most important reasons for the rapid progression of the HBV infection to OBI-associated cirrhosis.45, 46
In the current study, 12.1% of the participants were HBs-Ag positive, and also 4.7% and 3.7% had detectable HBV-DNA in PBMCs and plasma, respectively. Since the rate of HBV infection progression to chronic phase in HIV-positive people is four times higher than people without HIV, the risk of OBI is increased, as well.47
Occult HBV infection has been detected in various populations worldwide, for instance, this infection has been diagnosed in subjects with normal plasma level of ALT (16%) in Korea,48 in a community-based population (18%) in North American,49 in residents of two communities (2.9%) in China,50 in hemodialysis patients (9.8%) in Turkey,51 in patients with cryptogenic liver disease (19%-31%),52 in individuals with HIV infection (0%-89%),53-56 and in blood donors (1%-3%).52
This infection (OBI) has been found in two groups of Iranians. In three studies, its rate in HBsAg-negative and HBV DNA-positive HIV-infected patients has reported 2.2%,57 18.0%,58 and 58.2%59 and also in various studies, in HBsAg-negative, anti-HBcAb-positive, and HBV DNA-positive HIV-infected patients has announced 12.1% and 13.6%.60-62
In this study, the prevalence of OBI in HIV-positive participants was 3.1%. Occult HBV infection in HIV-positive patients is important, the immune-suppressed conditions in these patients can change OBI to overt HBV infection and also to HCC.63
Laboratory parameters of the studied subjects with HIV infection showed a significant increase in liver enzymes (ALT and AST). Coinfection of viral hepatitis and HIV can accelerate liver fibrosis progression and HCC can increase liver enzymes, which are known as bad prognosis.64 The evaluated ALT level is representative of liver damage. A study in Iran showed the increased level of ALT in patients with OCI.26 In Thailand, the high prevalence of HBV and HCV infection in HIV-infected Thai patients has reported. Male sex, elevated serum ALT levels, and low CD4 percentage are associated with coinfection of HBV and HCV and HIV.65 In this study, there was a significant association between the male sex and evaluated liver enzymes with HIV infection.
Due to the global cultural and demographical parameters as well as in the present study, male sex was found with a significant association with HIV infection66 except for sexual partners of IDU, who were discovered with a significant association regarding female sex; however, the trace of male sex in this parameter is demystifying.
Due to the lack of large-scale population studies, the exact prevalence of coinfection of HBV/HCV in the world has not yet known; however, there are some studies conducted all over the world, in which the coinfection rate of HBV/HCV has estimated about 0.7% to 15%.67 This coinfection in HIV-positive individuals can lead to higher rates of liver fibrosis.68
Interestingly, in the present study, two studied patients (1.1%) had OCI/OBI coinfection (Table 3). To the best of our knowledge, the present survey is the first study investigating the frequency of OBI and OCI in HIV-infected patients. It is noteworthy that there are numerous reports on the presence of OBI and OCI in various groups of populations worldwide, whereas there is no report on the detection of these infections in patients with HIV concurrently. According to the information obtained in the present study, it seems that this issue should be investigated further with a larger sample size and among various populations.
It is noteworthy that we did not have access to the patients' records, so we did not know the hepatic status of patients who were infected with HCV, HBV, HCV/HBV, OCI, OBI, or OCI/OBI. In conclusion, this study demonstrated that 1.1% of the HIV-infected people had OBI/OCI at the same time, 11.4% had OCI, and also more than 3% of the studied participants had OBI. Therefore, in addition to the routine tests to detect various infectious diseases in these patients, appropriate tests to diagnose OBI and OCI are valuable and informative and should be considered for these patients.
ACKNOWLEDGMENTS
The authors of the present survey would like to thank all the participants who enrolled in this cross-sectional research. This project was financially supported by Research Deputy of Iran University of Medical Sciences (Grant Number: 33167).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Ethical approval for the current research was obtained from the IUMS's Ethics Committee of the Gastrointestinal and Liver Disease Research Centre (the ethical code: IR.IUMS.REC 1397.1015). A ll the study population were informed about this study, and a written informed consent was obtained from all the individuals participated in the current study.




