Platelet activation during chronic hepatitis B infection exacerbates liver inflammation and promotes fibrosis
Richeng Mao is the co-first author.
Abstract
Recurrent hepatitis activity during chronic hepatitis B virus infection results in fibrosis and even hepatocellular carcinoma. It is still unclear what causes acute exacerbation. As platelets have recently been identified as a significant role in inflammation, we here investigated the role of platelets in mediating liver damage in patients with chronic hepatitis B virus infection. Platelet aggregation testing and flow cytometry were carried out to evaluate platelet activation status in 121 patients chronically infected with hepatitis B across different phases of the condition. The correlation between platelet aggregation rate and liver inflammation or liver fibrosis index was evaluated. To investigate the genesis of platelet activation, several serum cytokines were also assessed by MILLIPLEX microsphere-based multiplex cytokine assay. Active hepatitis patients showed a higher aggregation rate than others. Levels of CD62p, a marker of platelet activation, were also increased in this group of patients. Positive correlations between platelet aggregation rate and liver inflammation or liver fibrosis were also noted, indicating a significant role of platelet in the progression of liver disease. The level of tumor necrosis factor-alpha, which is known to trigger platelet activation, was markedly higher in the active hepatitis group (P < .005). Based on the findings in our study, platelet activation plays a vital role in the progression of chronic hepatitis B virus infection. Antiplatelet therapy may provide a new means of hepatitis B infection treatment.
Abbreviations
-
- ADP
-
- adenosine diphosphate
-
- ALT
-
- alanine aminotransferase
-
- APRI
-
- aspartate aminotransferase to platelet ratio index
-
- CHB
-
- chronic hepatitis B
-
- CTLs
-
- cytotoxic lymphocytes
-
- ENEG
-
- HBeAg-negative hepatitis
-
- HBV
-
- hepatitis B virus
-
- IA
-
- immune active
-
- IC
-
- inactive carrier
-
- IT
-
- immune tolerant
-
- NAs
-
- nucleotide/nucleoside analogs
-
- PDGFB
-
- platelet-derived growth factor-beta
-
- PPP
-
- platelet-poor plasma
-
- PRP
-
- platelet-rich plasma
-
- PSGL-1
-
- P-selectin glycoprotein ligand-1
-
- TNF-alpha
-
- tumor necrosis factor-alpha
1 INTRODUCTION
Globally, over 250 million people are living with chronic hepatitis B virus (HBV) infection. Left untreated, 20% or more of chronic HBV infection (CHB) patients will develop such life-threatening complications as cirrhosis and hepatocellular carcinoma.1 According to current practice guidelines, CHB can be divided into four phases, which include the immune-tolerant (IT) phase, the immune-active (IA) phase, the inactive carrier (IC) phase, and HBeAg-negative hepatitis (ENEG) phase.2 The IA and ENEG phases were considered to have active hepatitis and meet the criteria for therapy. Acute exacerbation may occur spontaneously during these two phases and advanced liver fibrosis progression can be more frequently observed than other phases.3, 4 There is still no definite mechanism to explain the development of acute exacerbation. As HBV is a noncytopathic virus, liver damage is believed to be immune-mediated. HBV-specific CD8+ T cells have conventionally been thought to be the main contributors to the pathology and outcome of chronic hepatitis B.5-7 Other immune cells, such as neutrophils and macrophages, can be recruited to the liver and aggravate liver injuries.8, 9
Recent studies in animals have further revealed that platelets enhance the accumulation of CD8+ T cells in the liver. Circulating CD8+ T cells are initially arrested within liver sinusoids by docking onto platelets. This allows these T cells to search for HBsAg-expressing hepatocytes and play their role as cytotoxic T cells.10 Moreover, diverse bioactive molecules released by activated platelets recruit inflammatory cells to the liver and drive different hepatic processes ranging from necroinflammation to fibrosis.11, 12 Apart from driving immune-mediated liver damage, platelets also decrease sinusoidal microcirculation by secreting serotonin and aggregating immunopathological liver cell damage.11, 13, 14 Platelet-derived growth factor-beta (PDGF-B) released by activated platelets activates hepatic stellate cells and accelerates the process of liver cirrhosis.12 Blocking platelet activation using aspirin or clopidogrel reduces the accumulation of virus-specific CD8+ T cells and virus-nonspecific inflammatory cells. This decreases the severity of fibrosis and delays the development of HCC.15, 16 However, no such phenomena have been confirmed in CHB patients across different phases of the condition.
In this study, we set out to determine whether platelet activation status varies with changing CHB phases. We also investigated the association between platelet activation, liver inflammation, and fibrosis to explore the function of platelets in the pathogenesis of acute exacerbation of CHB. Chemokine levels were measured to analyze the possible pathogenic mechanisms of platelet activation.
2 METHODS
2.1 Patients
Consecutive participants from April 2018 to April 2019 were included after they gave written informed consent at Huashan Hospital, Fudan University. The study was conducted according to the principles stated in the Declaration of Helsinki and was also approved by the Institutional Ethics Committee, Huashan Hospital, Fudan University, Shanghai, China.
All the patients included were positive for hepatitis B surface antigen (HBsAg) for over 6 months. Patients who were treatment-naïve, aged 18 years or above, were recruited. Patients were grouped according to the clinical phases of chronic HBV infection: IT, IA, IC, and ENEG, based on the 2017 European Association for the Study of the Liver (EASL) guidelines.2, 17
Exclusion criteria were (a) previous treatment with interferon-alpha or currently undergoing treatment with nucleoside/nucleotide analogs; (b) diagnosed with hepatitis C, hepatocellular carcinoma or other nonrelated cancers, cholestatic liver disease, vascular atherosclerotic diseases, acute hepatitis, or other acute infections; (c) treatment with antibiotics, aspirin, or other nonsteroidal anti-inflammatory drugs in the previous 30 days; (d) recent need of transfusion of platelets or plasma; (e) complicated with extrahepatic malignancy; (f) active alcohol intake in the last 6 months; and (g) pregnancy or breast-feeding.
Healthy volunteers age 18 or older were also included as controls in this study. Controls were defined as healthy if they did not meet the exclusion criteria described above and did not have a diagnosis of CHB.
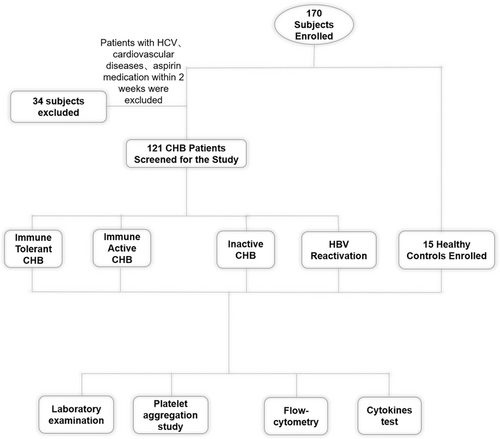
A flowchart describing cohort recruitment and study design is shown in Figure 1.
2.2 Collection of laboratory data
Qualitative serum HBsAg, antibodies to HBsAg (anti-HBs), HBeAg, and anti-HBe, were tested using commercial assays (Abbott, North Chicago, IL). Serum HBV DNA titer was measured by the Amplicor assay (Roche Molecular Systems, Branchburg, NJ). Serum ALT (alanine aminotransferase) was detected on an automated analyzer at the diagnostic center of Huashan Hospital. The aspartate aminotransferase to platelet ratio index (APRI) was calculated using the formula 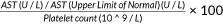 .
.
2.3 Sample preparation
Blood samples were collected into a vacutainer tube by venipuncture using a 20-gauge needle. To avoid activated platelet contamination, the first part of the sample (2 mL) was discarded. After the tourniquet was released, the blood was collected into a vacutainer tube containing ACD (85 mM sodium citrate, 71.38 mM citric acid, and 27.78 mM glucose) as an anticoagulant. To obtain platelet-rich plasma (PRP), the collected blood was centrifuged at 300 g for 8 minutes. PRP was then removed to a clean tube without disturbing the buffy coat. Platelet-poor plasma (PPP) was separated by centrifugation of the remaining blood at 1500 g for a further 10 minutes. The platelet count was adjusted to the range of 2 to 6 × 108/mL using PPP.18 The remaining PPP was then harvested and stored at −80°C until it was used for cytokine tests.
2.4 Platelet aggregation test
The platelet aggregation test was carried out on a lumi-aggregometer (Model 400VS; Chrono-Log, Haverston, PA) under stirring conditions (900 rpm) at 37°C as reported previously.19-22 To set the aggregation scale, platelet-poor plasma was used before each study. PRP from patients and healthy volunteers was exposed to adenosine diphosphate (ADP) (5 μM, Sigma-Aldrich, St. Louis, MO). The aggregation tracings were recorded using a recorder (Model 707; Chrono-Log) connected to the aggregometer. Aggregation results, shown as % increase in light transmission, were measured 5 minutes after agonist addition. Results are shown as median and interquartile ranges. All experimentation was performed within 6 hours of preparation of the PRP, as described previously.23
2.5 Flow cytometry tests
Flow cytometry was carried out to investigate the platelet activation phase with and without agonist. Blood samples were those described above. Platelets were incubated for 20 minutes at room temperature with fluorescent-labeled monoclonal antibodies (5 µL) and 10 μM ADP (Stago, Asnières, France) within 5 minutes of sample collection. The antibodies used were: CD42b (catalog number 303903, Clone HIP1; Biolegend), CD62p (catalog number 304910, Clone AK4; Biolegend), and Fixable Viability Dye (catalog number 65-0863-14; eBioscience) according to the manufacturers' instructions.
Samples were analyzed on a Cytoflex S flow cytometer (Beckman Coulter, Fullerton, CA) at the Huashan Hospital. Fixed samples were analyzed within 6 hours of processing. Platelets were gated on the basis of CD42b and sideward scatter. The percentage of platelets expressing CD62p was then recorded.
2.6 Assessment of cytokine levels using multiplex immunoassays
Serum was collected as described above and stored at −80°C until testing. Concentrations of 25 distinct analytes were measured using the MILLIPLEX Bead-Based Multiplex Assays using Luminex technology according to the manufacturer's instructions. Cytokines tested in our study were interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17A, IL-17E/IL-25, IL-17F, IL-21, IL-22, IL-23, IL-27, IL-28A, IL-31, IL-33, granulocyte-macrophage colony-stimulating factor, interferon gamma, macrophage inflammatory protein-3 alpha, TNF-α, and TNFβ.
2.7 Statistical analysis
Skewed quantitative data are here expressed as median and interquartile ranges, as indicated. Pairs of groups were compared using an unpaired Student t test or Mann–Whitney U test. Sets of three or more groups were compared using a one-way analysis of variance (ANOVA) or Kruskal–Wallis test. Besides, Bonferroni corrected post-hoc tests were conducted to adjust the observed significance level for multiple comparisons if the null hypothesis was rejected. Associations between aggregation rate and clinical variables were examined by Pearson's correlation or Spearman's rank correlation test. Values of P < .05 were considered statistically significant. All analyses were performed with GraphPad Prism version 6.0 software (GraphPad Software, La Jolla, CA).
3 RESULTS
3.1 Clinical characteristics of participants
There were 121 patients diagnosed with CHB who fulfilled the inclusion criteria. These patients were enrolled and divided into four groups according to the natural history of chronic HBV infection.2 Patients' baseline characteristics are summarized in Table 1. When compared with healthy subjects and those without active hepatitis, patients undergoing active hepatitis had higher levels of ALT, which is a major marker of hepatocyte injury. APRI was also higher in the active phase groups than in the remaining groups. Platelet counts did not differ among groups.
| Demographic characteristics | NC (n = 15) | IT (n = 16) | IA (n = 50) | IC (n = 42) | ENEG (n = 13) |
|---|---|---|---|---|---|
| Male gender, n (%) | 3/15 | 7/17 | 28/46 | 24/42 | 10/13 |
| Age, y mean ± SD | 25 (24,27) | 27 (26, 31) | 33 (28, 43) | 41 (34, 48) | 44 (32, 51) |
| Platelet count (x10^9/L) | N.A. | 212 (184, 257) | 176 (146, 226) | 184 (131, 231) | 184 (119, 255) |
| ALT, U/L | N.A. | 26 (21, 33) | 75 (47, 114) | 22 (18, 27) | 75 (58, 186) |
| AST, U/L | N.A. | 20 (17, 24) | 45 (31, 86) | 24 (19, 27) | 49 (38, 142) |
| TBIL, μmol/L | N.A. | 11.8 (7.9, 13.9) | 12.6 (11.4, 16.1) | 13.9 (9.6, 17.7) | 14.1 (10.7, 15.6) |
| Log10 HBV DNA (IU/mL) | N.A. | 7.52 (7.48, 7.60) | 6.77 (4.94, 7.48) | 0 (0, 2.98) | 5.21 (2.41, 6.67) |
| HBsAg, S/CO | N.A. | 52000 (52000, 52000) | 8174 (1193, 22910) | 1432 (294, 4001) | 2853 (632, 4974) |
| Fibrosis index | |||||
| APRI | N.A. | 0.21 (0.17, 0.24) | 0.55 (0.39, 1.30) | 0.28 (0.23, 0.39) | 1.1 (0.4, 1.9) |
| Aggregation rate (%) | 38 (32, 42) | 40 (30, 45) | 45 (43, 50) | 38 (32, 42) | 46 (44, 51) |
- Note: Values are presented as median with IQR.
- Abbreviations: ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate transaminase; ENEG, e Ag negative hepatitis; HBV, hepatitis B virus; IA, immune active; IC, inactive carrier; IQR, interquartile range; IT, immune tolerant; NC, normal control; TBIL, total bilirubin.
3.2 Elevated platelet aggregation in patients with active hepatitis
The overall platelet aggregation rate in the CHB patient group was higher than that in healthy controls (Figure 2B). In detail, the percentages were as follows: NC, 38% (range 32%–42%); IT, 40% (range 30%–45%); IA, 45% (range 43%–50%); IC, 38% (range 32%–42%); and ENEG, 45% (range 44%–51%) (Figure 2C). The platelet aggregation rates in patients with active hepatitis were higher than in those with an inactive disease or normal controls.
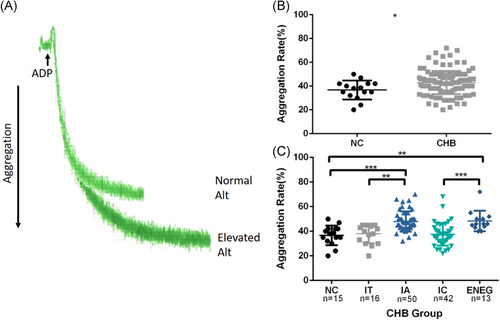
3.3 Increased CD62p levels in patients with active hepatitis
CD62p (also called P-selectin) is known to be expressed on activated platelets and can be used as a marker to test platelet activation. In this study, the percentage of platelets positive for CD62p in samples without stimulation was equally low for all samples (≤ 4%). Levels of CD62p expression on platelets in ADP-stimulated samples were higher in patients with active hepatitis than in healthy individuals or in those without biochemical liver damage (Figure 3).
3.4 The positive relationship between platelet activation and biological parameters for liver inflammation and fibrosis
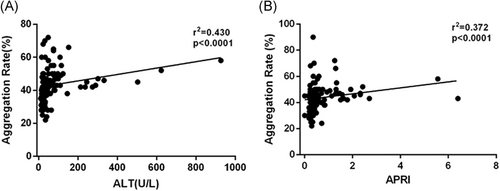
We noticed that platelet aggregation differed among different disease phases, so we set out to determine the correlation between the level of platelet aggregation, liver damage index, and fibrosis index. There was a positive correlation between platelet aggregation rate and ALT, a marker of liver inflammation (r = 0.430, P < .001), and also a positive correlation between platelet aggregation rate and APRI, a noninvasive liver fibrosis index (r = 0.372, P < .001) (Figure 4).
3.5 Increased pro-inflammatory cytokines in patients with active hepatitis
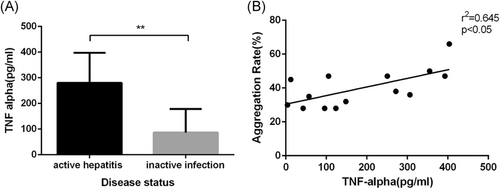
To explore the mechanism mediating the differences in platelet activation among the different groups and the correlations between liver damage indexes, we tested multiple proinflammatory cytokines using a MILLIPLEX. Most of the cytokines we tested in our study were higher in the active hepatitis groups than in those without active hepatitis or in healthy subjects (Data not shown). Among these, tumor necrosis factor-alpha (TNF-alpha) was markedly increased (P < .005) and a positive correlation between platelet aggregation rate and the level of TNF-alpha could be noted. (Figure 5).
4 DISCUSSION
Recurrent inflammation caused by chronic HBV infection appears to play a significant role in accelerating continuous cycles of hepatocellular death and compensatory liver cell proliferation. These cycles, over time, are thought to intensify liver fibrosis and multiple genetic alterations associated with HCC.5, 24 Because HBV-specific immune cells and nonspecific inflammatory cells mainly contributed to the hepatocyte damage, an effective means of preventing recurrent liver inflammation from reducing the recruitment of inflammatory cells is urgently needed. There have been several studies in mice that have revealed that inhibitors of platelet activation modulate platelet-dependent intrahepatic accumulation of virus-specific cytotoxic lymphocytes (CTLs) and reduce the level of ALT, which is a direct marker to identify hepatocytes damage.15, 16 In this study, we found that the activation of platelets is more pronounced with some disease phases, suggesting that platelets tend to be more easily activated during specific phases of CHB. In addition, we analyzed the correlation between platelet aggregation rate and ALT and found that there is a positive correlation. This indicates that activated platelets may directly involve in the immunological damage of hepatitis B. Platelets possess a vast array of immune receptors and inflammatory molecules.25-27 This occurs as platelets both supply and respond to signals early during the immune process.28 Enhanced expression of CD62p on the surface of platelets during active hepatitis was also noticed in our study. The primary ligand of CD62p is P-selectin glycoprotein ligand-1 (PSGL-1), which is expressed on most leukocytes29 and plays a vital role in recruiting white blood cells into the inflamed tissues. By promoting the recruitment of CD8+T cells, dendritic cells, and other immune cells, platelets contribute to the initiation of liver inflammation, thus amplifying liver damage. In addition to amplifying liver damage, bioactive proteins released by activated platelets also instigate liver fibrosis.12 In our study, a positive correlation between platelet aggregation rate and APRI, which is known to be a noninvasive liver fibrosis index, is consistent with those in mice.
To determine the role of platelets in amplifying liver inflammation, we wondered whether platelets are activated by the process of acute hepatitis. It has been reported that leukocytes and many cytokines lead to platelet activation in other diseases.30-35 In our study, we found that TNF-alpha was elevated in active hepatitis. We also discovered a positive correlation between platelet aggregation rate and TNF-alpha level. TNF-alpha was reported to result in platelet clumping and activation.36, 37 Active hepatitis also causes liver sinusoidal endothelial damage, which also activates platelets. Activated platelets then participate in liver inflammation and contribute to liver fibrosis.
Results showed that the changes in platelet function play important roles in the occurrence of active hepatitis in chronic hepatitis B and in the development of the progressive fibrotic process. The current treatment strategy for CHB patients involves nucleotide/nucleoside analogs (NAs) or with interferon-alpha.2 As given in the current clinical practice guideline, long-term NAs therapy has been shown to reverse liver inflammation and fibrosis. However, some patients who achieved HBV suppression after NAs treatment can still progress to cirrhosis.2, 38 There have been several reports that revealed that aspirin, a known platelet activation inhibitor, could reduce progression to liver fibrosis and even HCC.16, 39 Therefore, it is advisable to consider antiplatelet therapy as a combination therapy to reduce the severity of liver damage and to decrease the development of liver fibrosis and HCC.15, 40 Moreover, our correlation data suggest that platelet aggregation tests may be indicative of disease activity and fibrosis development. From our study, we postulate that aspirin may inhibit liver cirrhosis by inhibiting platelet activation. Further studies focusing on this area are needed.
The limitations of this study are the small sample size, and therefore studies with a large sample size are needed to confirm our findings. Our study was mainly carried out by studying platelet aggregation, which is used as the gold standard test for platelet activation. Only a low volume is needed for flow cytometry tests, so this opens up the possibility of exploring the relationship between platelets and other immune cells directly.
Overall, our study shows that platelet activation plays an indispensable role in the progression of CHB. Platelet activation may be considered as an additional target in developing hepatitis therapeutics or improving the effectiveness of current therapies.
ACKNOWLEDGMENTS
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. The present study was supported by the National Natural Science Foundation of China (81670528, 81672009, and 81871640), National “13th five-year plan” Major Infectious Disease Project (No. 2017ZX10202203007 and No. 2017ZX10202202) and the Shanghai Pujiang Pujiang Program (17PJD005).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
The respective roles of each author are as follows. QJ, LC, and RM were responsible for experiment and data collection, HZ, GZ and JW were responsible for clinical data analysis. JZ and ZD were responsible for study design. QJ and JZ wrote the manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethical committee of Huashan Hospital, Fudan University, (2018–19). All subjects gave informed consent for their participation in the study.




