Impact of ribavirin dosage in chronic hepatitis C patients treated with simeprevir, pegylated interferon plus ribavirin combination therapy
Abstract
The factors associated with sustained virologic response (SVR) in chronic hepatitis C (CH-C) genotype 1 patients treated with simeprevir (SMV), pegylated interferon (Peg-IFN) plus ribavirin (RBV) triple therapy have not been fully investigated. Two hundred and twenty-nine treatment-naïve CH-C patients treated with SMV triple therapy were enrolled in this study. The overall SVR rate was 87% in per-protocol analysis. In multivariate analysis, the interleukin (IL) 28B genotype (rs8099917, TT vs. non-TT, odds ratio [OR]: 0.044, P = 0.001) and RBV dose (< 10/10–12/ ≥ 12 mg/kg/day, OR: 4.513, P = 0.041) were significant factors associated with SVR. In patients with the IL28B non-TT genotype, RBV dose affected SVR dose-dependently in stratified analysis of RBV dose (P = 0.015); it was 44% (8/18) for patients administered <10 mg/kg/day of RBV, 78% (14/18) for those administered 10–12 mg/kg/day of RBV, and 100% (3/3) for those administered ≥12 mg/kg/day of RBV, whereas in patients with the IL28B TT genotype, a significant correlation between SVR and RBV dose was not observed (P = 0.229). Regarding RBV dose reduction of less than 10 mg/kg/day, the inosine triphosphate pyrophosphatase (ITPA) genotype (rs1127354, CC vs. non-CC, OR: 0.239, P = 0.003) and age (by 1 y.o., OR: 1.084, P = 0.002) were significant independent factors. RBV dosage affected SVR dose-dependently in patients with the IL28B non-TT genotype treated with SMV triple therapy. Special attention to anemia progression and RBV dosage should be paid to aged patients with the ITPA CC genotype. J. Med. Virol. 88:1776–1784, 2016. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Simeprevir (SMV), pegylated interferon (Peg-IFN) plus ribavirin (RBV) combination therapy is the first line of interferon (IFN)-based therapies and is recommended for patients with chronic hepatitis C (CH-C) genotype 1 in Japan and Europe [Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology, 2014; European Association for the Study of the Liver, 2014]. Factors associated with the treatment effect include interleukin (IL) 28B genotype, previous treatment effect, and liver fibrosis; these were reported to be associated with sustained virologic response (SVR) in clinical trials of SMV, Peg-IFN plus RBV combination therapy for patients with chronic hepatitis C virus (HCV) infections [Forns et al., 2014; Hayashi et al., 2014; Izumi et al., 2014; Jacobson et al., 2014; Manns et al., 2014; Kumada et al., 2015].
In recent years, the IL28B genotype has been reported to be strongly associated with treatment effects in Peg-IFN plus RBV combination therapy [Ge et al., 2009; Tanaka et al., 2009]; SVR rate was higher in patients with the IL28B major genotype (rs8099917; TT, rs12979860; CC) than in those with the IL28B minor genotype (rs8099917; TG/GG, rs12979860; CT/TT). In clinical trials of SMV, Peg-IFN plus RBV combination therapy for treatment-naïve patients, the IL28B genotype was reported to be associated with SVR [Hayashi et al., 2014; Jacobson et al., 2014; Manns et al., 2014]; the SVR rates were 94–96% in patients with the IL28B major genotype and were significantly higher than in those with the IL28B minor genotype.
Peg-IFN plus RBV combination therapy was a main treatment regimen for CH-C patients before the development of direct acting antivirals (DAAs). Indeed, the combination therapy with DAA and Peg-IFN plus RBV has increased the treatment effect, but the adjustment of drug dose is essential for the improvement of the treatment effect. In the era of Peg-IFN plus RBV combination therapy, the Peg-IFN dose was reported to raise the complete early virologic response (c-EVR) rate, which indicated HCV-RNA negativity at week 12 dose-dependently [Oze et al., 2009]. Furthermore, the RBV dose reduction was reported to raise post-treatment relapse dose-dependently [Hiramatsu et al., 2009]. In telaprevir (TVR), Peg-IFN plus RBV combination therapy, the adjustment of TVR dose affected the safety and efficacy of this therapy. An increase of the TVR dose led to an increase in the incidence of adverse effects, whereas a reduction of the TVR dose led to a decrease in treatment effects. In previous study [Oze et al., 2015], patients administered 25–35 mg/kg/day of TVR achieved the highest SVR rate (91%) compared with those administered <25 mg/kg/day (71%) or ≥35 mg/kg/day (78%). On the other hand, in SMV, Peg-IFN plus RBV combination therapy, the SMV dose was fixed, and SMV had few adverse effects. Therefore, an adjustment of the Peg-IFN and the RBV dose is very important for increasing the treatment effect in SMV, Peg-IFN plus RBV combination therapy.
In the present study, the factors that are difficult to treat and the influence of drug adherence on the treatment effect in SMV, Peg-IFN plus RBV combination therapy were examined.
PATIENTS AND METHODS
Study Patients
This study was a prospective, multicenter study that was performed by Osaka University Hospital and other institutions attending the Osaka Liver Forum. This study included a total of 229 consecutive treatment-naïve patients with chronic HCV genotype 1 infection who began SMV, Peg-IFN plus RBV combination therapy between December 2013 and July 2014.
Patients over 20 years old, infected with HCV genotype 1 and with a high viral load (≥105 IU/ml) were included in this study. Exclusion criteria were decompensated cirrhosis, liver failure, hepatocellular carcinoma, co-infection with human immunodeficiency virus or hepatitis B virus, other causes of liver disease (e.g., autoimmune hepatitis or drug-induced liver disease), and comorbid disease such as depression, immunodeficiency, or chronic kidney disease. Informed consent was given and written consent was obtained from all patients before participation. This study was conducted in compliance with the ethical guidelines of the 1975 Declaration of Helsinki, which was amended in 2002 and was approved by the independent ethics committee of Osaka University Hospital and institutional review boards of all study centers attending the Osaka Liver Forum (UMIN000012183).
Treatment Protocol
All patients were treated in accordance with a standard therapeutic course in Japan. Patients received SMV (SOVRIAD; Janssen Pharmaceutical K. K., Tokyo, Japan) for the first 12 weeks and combination therapy of Peg-IFN α-2a (PEGASYS; Chugai Pharmaceutical Co. Ltd., Tokyo, Japan) plus RBV (COPEGUS; Chugai Pharmaceutical Co. Ltd.) or Peg-IFN α-2b (PEGINTRON; MSD K.K., Tokyo, Japan) plus RBV (REBETOL; MSD K.K.) for 24 weeks. SMV was administered by mouth with doses of 100 mg once per day. As a rule, Peg-IFN α-2a was administered subcutaneously with doses of 180 μg once per week and Peg-IFN α-2b was administered subcutaneously with doses between 60 μg and 150 μg based on patients' body weight once per week (patients 35–45 kg were given 60 μg, patients 46–60 kg given 80 μg, patients 61–75 kg given 100 μg, patients 76–90 kg given 120 μg, patients 91–120 kg given 150 μg). RBV was administered by mouth with doses between 600 mg and 1000 mg based on patients' body weight twice per day (patients 60 kg or less were given 600 mg, patients 60–80 kg given 800 mg, patients more than 80 kg given 1,000 mg). In 13 patients, the RBV dose was reduced because of the judgment by an attending doctor at the start.
Dose Adjustments
As a rule, dose adjustments of each drugs were conducted following the manufacturers' information. Peg-IFN α-2a dose was decreased to 50% of the original dose when the platelet (Plt) count dropped to <5 × 104/mm3 or when the neutrophil count dropped to <750/mm3. Peg-IFN α-2b dose was decreased to 50% of the original dose when the Plt count dropped to <8 × 104/mm3, when the white blood cell (WBC) count dropped to <1,500/mm3, or when the neutrophil count dropped to <750/mm3. RBV dose was decreased from 1,000 to 600, from 800 to 600, or from 600 to 400 mg when the hemoglobin (Hb) level dropped to <10 g/dl. Peg-IFN α-2a and RBV were interrupted when the Plt count dropped to <2.5 × 104/mm3, when the neutrophil count dropped to <500 /mm3, or when the Hb level dropped to <8.5 g/dl. Peg-IFNα-2b and RBV were interrupted when the Plt count dropped to <5 × 104/mm3, when the WBC count dropped to <1,000/mm3, when the neutrophil count dropped to <500/mm3, or when the Hb level dropped to <8.5 g/dl.
Assessment of the Virologic Response
Serum HCV-RNA levels were measured by using the COBAS Taqman HCV test, ver. 2.0 (quantitative range 1.2–7.8 log10 IU/ml; Roche Diagnostics, Branchburg, NJ). Serum HCV-RNA levels were monitored at baseline, day 2, weeks 1, 2, every four weeks from weeks 4 to 24 during treatment and 12 weeks after the end of treatment (EOT). SVR was defined as a serum HCV-RNA negativity at 12 weeks after the EOT (SVR12).
Drug Adherence
The real doses of SMV, Peg-IFN, and RBV that were administered to each patient during treatment were calculated by checking medical records. The mean doses of each drug were evaluated based on patients' body weight at baseline and divided by actual treatment period. SMV dose and RBV dose were indicated as mg/kg/day and Peg-IFN dose was indicated as μg/kg/week.
Histological Evaluation
Liver biopsy was conducted before starting SMV, Peg-IFN plus RBV combination therapy. The grade of activity and fibrosis of liver specimens were evaluated and scored in accordance with the METAVIR histological system by experienced liver pathologists [Bedossa and Poynard, 1996].
Safety Assessment
Safety assessments including blood tests were performed every week from the start of treatment to week 8, and every four weeks from week 8 to the EOT. If attending doctors evaluated the clinical need for a safety assessment, blood tests and physical examinations were conducted and data of the adverse effects were collected.
IL28B Genotype and ITPA Genotype
Human genomic DNA was extracted from patients' peripheral blood. Genetic polymorphisms situated near the IL28B gene (rs8099917) and the inosine triphosphate pyrophosphatase (ITPA) gene (rs1127354) were decided by real-time PCR. The IL28B genotype and the ITPA genotype were analyzed by real-time PCR in a thermal cycler (7900 Real-time PCR System, Applied Biosystems, Foster city, CA). With respect to the IL28B genotype, homozygosity (TT genotype) was defined as a major genotype, whereas a non-TT genotype including heterozygosity (TG) or homozygosity (GG) was defined as a minor genotype. With respect to the ITPA genotype, homozygosity (CC genotype) was defined as a major genotype, whereas the non-CC genotype including heterozygosity (CA) or homozygosity (AA) was defined as a minor genotype.
Statistical Analysis
The baseline categorical variables were shown as frequencies, and the continuous variables were shown as the mean ± standard deviation or the median value. The analyses of the factors associated with the virologic response and RBV dose reduction were conducted per protocol. As for the factors associated with treatment outcome (SVR and RBV dose reduction), univariate analysis was conducted and subsequently multivariate analysis was conducted using logistic regression analysis. Baseline factors (age, sex, body mass index [BMI], degree of liver activity, degree of liver fibrosis, WBC count, neutrophil count, Hb level, Plt count, total bilirubin level, alanine aminotransferase [ALT] level, gamma-glutamyl transpeptidase [GGT] level, alpha-fetoprotein [AFP] level, IL28B genotype, and ITPA genotype) and drug adherence (mean SMV dose, mean Peg-IFNα-2a dose, mean Peg-IFNα-2b dose, and mean RBV dose) were used for analyses of the factors associated with SVR. Baseline factors were used for analyses of the factors associated with RBV dose reduction. The significant differences in the trends among categorical data were analyzed using Fisher's exact test or the Mantel Haenszel chi square test. A P-value <0.05 (two-tailed) was counted as statistically significant. SPSS ver. 22.0 (IBM, Armonk, NY) and SAS ver. 9.4 (SAS Institute, Inc., Cary, NC) were used for statistical analyses.
RESULTS
Baseline Characteristics of Study Patients
Table I shows baseline characteristics of the 229 patients in the present study. The mean age was 61.0 years old (y.o.), and 100 patients were male and 129 patients were female. The median value of HCV-RNA was 6.4 log10 IU/ml. The mean levels of Plt, total bilirubin, and ALT were 16.5 × 104/µl, 0.8 mg/dl, and 63 U/l, respectively. Regarding the IL28B genotype, 146 patients had the TT genotype, and 46 patients had the non-TT genotype. Regarding the ITPA genotype, 119 patients had the CC genotype and 52 patients had the non-CC genotype.
| Factor | n = 229 |
|---|---|
| Age (years old) | 61.0 ± 10.9 |
| Sex: male/female | 100/129 |
| BMI (kg/m2) | 22.8 ± 3.2 |
| HCV-RNA (median, log10 IU/ml) | 6.4 |
| Liver histology: Activity: A0/1/2/3: Fibrosis: F0/1/2/3/4 | 0/134/57/715/100/54/19/11 |
| White blood cell (/µl) | 5,031 ± 1,475 |
| Neutrophils (/µl) | 2,624 ± 1,022 |
| Hemoglobin (g/dl) | 13.9 ± 1.4 |
| Platelets (×104/µl) | 16.5 ± 5.6 |
| Total bilirubin (mg/dl) | 0.8 ± 0.3 |
| Direct bilirubin (mg/dl) | 0.2 ± 0.1 |
| AST (U/L) | 54 ± 34 |
| ALT (U/L) | 63 ± 46 |
| GGT (IU/L) | 52 ± 54 |
| Creatinine (mg/dl) | 0.7 ± 0.2 |
| IL28B genotype (rs8099917): TT/non-TT | 146/46 |
| ITPA genotype (rs1127354): CC/non-CC | 119/52 |
| Peg-IFNα: 2a/2b | 29/200 |
- BMI, body mass index; HCV, hepatitis C virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; IL28B, interleukin 28B; ITPA, inosine triphosphate pyrophosphatase; Peg-IFN, pegylated interferon.
Virologic Response
The analyses of the virologic response were conducted per protocol. Treatment was discontinued in 22 patients (rash: four patients, fatigue: three patients, psychological symptom: two patients, liver injury: two patients, bilirubin increase: one patient, depression: one patient, retinopathy: one patient, others: eight patients). Seven patients were lost to follow-up after the end of treatment in per protocol analyses. The mean HCV-RNA levels at baseline, day 2, week 2, and week 4 of treatment were 6.36 ± 0.70, 2.83 ± 0.75, 0.89 ± 0.79, and 0.19 ± 0.58 log10 IU/ml, respectively. The HCV-RNA negativity rates at week 2, 4, 12, and EOT were 34%, 86%, 98%, and 97%, respectively, and the SVR12 rate was 87%.
The Factors Associated With SVR Including Baseline Factors and Drug Adherence
Table II shows the factors associated with SVR12, including baseline factors and drug adherence. The multivariate analysis was conducted using eight factors identified as significant by the univariate analysis (the degree of liver fibrosis, WBC count, neutrophil count, Plt count, GGT level, AFP level, the IL28B genotype, and the mean RBV dose), and the IL28B genotype (TT vs. non-TT, odds ratio [OR]: 0.044, P = 0.001) and the mean RBV dose (< 10/10–12/ ≥ 12 mg/kg/day, OR: 4.508, P = 0.041) were selected as significant factors. The SVR12 rates according to the IL28B genotype were 95% (121/137) in patients with the IL28B TT genotype and 64% (25/39) in those with the IL28B non-TT genotype (Fig. 1A). The SVR12 rates were significantly higher in patients with the IL28B TT genotype than in those with the IL28B non-TT genotype (P < 0.001). The SVR12 rates according to the mean RBV dose were 77% (59/77) for patients administered <10 mg/kg/day of RBV, 91% (80/88) for those administered 10–12 mg/kg/day of RBV, and 100% (35/35) for those administered ≥12 mg/kg/day of RBV (Fig. 1B). The mean RBV dose produced a dose-dependently increase in SVR12 rate (P < 0.001).
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Category | OR | 95%CI | P-value | OR | 95%CI | P-value |
| Age (years old) | 0.986 | 0.948–1.026 | 0.500 | ||||
| Sex | Male/female | 0.560 | 0.231–1.356 | 0.199 | |||
| BMI (kg/m2) | 0.969 | 0.854–1.099 | 0.621 | ||||
| Activity | 0, 1/2, 3 | 0.634 | 0.251–1.606 | 0.337 | |||
| Fibrosis | 0–2/3, 4 | 0.333 | 0.115–0.969 | 0.044 | 0.477 | 0.043–5.261 | 0.545 |
| White blood cell (/μl) | 100/μl | 1.041 | 1.007–1.076 | 0.018 | 0.949 | 0.823–1.094 | 0.472 |
| Neutrophil (/μl) | 100/μl | 1.086 | 1.029–1.146 | 0.003 | 1.191 | 0.967–1.467 | 0.101 |
| Hemoglobin (g/dl) | 1.334 | 0.986–1.805 | 0.062 | ||||
| Platelets (×104/μl) | 1.160 | 1.057–1.274 | 0.002 | 1.144 | 0.907–1.443 | 0.255 | |
| Total bilirubin (mg/dl) | 0.492 | 0.146–1.661 | 0.253 | ||||
| ALT (U/L) | 0.998 | 0.989–1.006 | 0.599 | ||||
| GGT (IU/L) | 0.989 | 0.982–0.995 | 0.001 | 0.989 | 0.975–1.003 | 0.115 | |
| AFP (ng/dl) | 0.981 | 0.963–0.999 | 0.034 | 0.997 | 0.959–1.036 | 0.866 | |
| IL28B genotype | TT/non-TT | 0.089 | 0.031–0.253 | <0.001 | 0.044 | 0.007–0.273 | 0.001 |
| ITPA genotype | CC/non-CC | 1.853 | 0.580–5.923 | 0.298 | |||
| SMV dose (mg/kg/day) | <1.43/1.43–2/≥2 | 1.003 | 0.523–1.923 | 0.992 | |||
| Peg-IFNα 2a dose (μg/kg/w) | <2/2–3/≥3 | 7.474 | 0.555–100.566 | 0.129 | |||
| Peg-IFNα 2b dose (μg/kg/w) | <1.2/1.2–1.5/≥1.5 | 1.802 | 0.994–3.266 | 0.052 | |||
| RBV dose (mg/kg/day) | <10/10–12/≥12 | 3.959 | 1.800–8.707 | 0.001 | 4.508 | 1.062–19.133 | 0.041 |
- BMI, body mass index; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; AFP, alpha-fetoprotein; IL28B, interleukin 28B; ITPA, inosine triphosphate pyrophosphatase; SMV, simeprevir; Peg-IFN, pegylated interferon; RBV, ribavirin; OR, odds ratio.
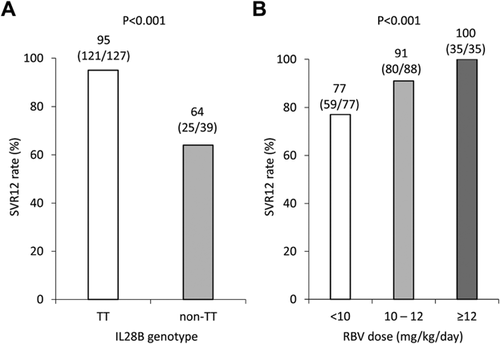
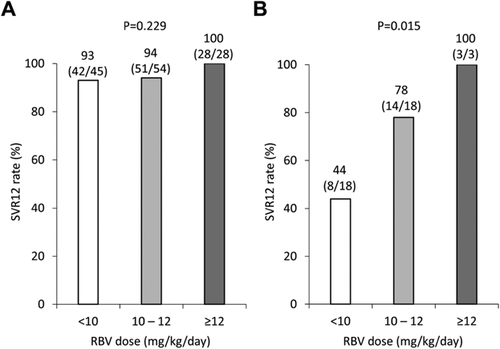
The Impact of the IL28B Genotype and RBV Dose During Treatment on SVR
Figure 2 shows the SVR12 rate according to the IL28B genotype and the mean RBV dose. In patients with the IL28B TT genotype, the SVR12 rates were 93% (42/45) for patients administered <10 mg/kg/day of RBV, 94% (51/54) for those administered 10–12 mg/kg/day of RBV, and 100% (28/28) for those administered ≥12 mg/kg/day of RBV, and the mean RBV dose was not significantly associated with the SVR12 rate (P = 0.229) (Fig. 2A). However, in patients with the IL28B non-TT genotype, the SVR12 rate was 44% (8/18) for patients administered <10 mg/kg/day/ of RBV, 78% (14/18) for those administered 10–12 mg/kg/day of RBV, and 100% (3/3) for those administered ≥12 mg/kg/day of RBV, and the mean RBV dose was significantly associated with the SVR12 rate dose-dependently (P = 0.015) (Fig. 2B).
The Factors Associated With RBV Dose Reduction
Next, the factors associated with the RBV dose reduction (RBV less than 10 mg/kg/day) after the start of treatment were investigated. Table III shows the factors associated with the RBV dose reduction (RBV less than 10 mg/kg/day) in the baseline factors, except for 13 patients whose RBV dose was reduced at the start. The multivariate analysis was conducted by using five factors identified as significant by the univariate analysis (age, sex, Hb level, Plt count, and the ITPA genotype). As a result, age (by 1 y.o., OR: 1.084, P = 0.002) and the ITPA genotype (CC vs. non-CC, OR: 0.239, P = 0.003) were selected as significant factors.
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Category | OR | 95%CI | P-value | OR | 95%CI | P-value |
| Age (years old) | 1.061 | 1.026–1.097 | <0.001 | 1.084 | 1.031–1.141 | 0.002 | |
| Sex | Male/female | 1.877 | 1.019–3.456 | 0.043 | 0.935 | 0.404–2.167 | 0.876 |
| BMI (kg/m2) | 0.988 | 0.902–1.083 | 0.802 | ||||
| Activity | 0, 1/2, 3 | 1.014 | 0.523–1.966 | 0.967 | |||
| Fibrosis | 0–2/3, 4 | 1.171 | 0.470–2.917 | 0.735 | |||
| White blood cell (/μl) | 100/μl | 0.980 | 0.960–1.001 | 0.062 | |||
| Neutrophil (/μl) | 100/μl | 0.981 | 0.952–1.011 | 0.219 | |||
| Hemoglobin (g/dl) | 0.631 | 0.494–0.805 | <0.001 | 0.933 | 0.666–1.307 | 0.688 | |
| Platelets (×104/μl) | 0.935 | 0.884–0.990 | 0.020 | 0.988 | 0.914–1.069 | 0.768 | |
| Total bilirubin (mg/dl) | 0.577 | 0.220–1.511 | 0.263 | ||||
| ALT (U/L) | 0.999 | 0.992–1.005 | 0.683 | ||||
| GGT (IU/L) | 1.002 | 0.996–1.007 | 0.559 | ||||
| AFP (ng/dl) | 0.998 | 0.983–1.014 | 0.829 | ||||
| IL28B genotype | TT/non-TT | 2.024 | 0.965–4.244 | 0.062 | |||
| ITPA genotype | CC/non-CC | 0.279 | 0.113–0.687 | 0.005 | 0.239 | 0.092–0.619 | 0.003 |
- BMI, body mass index; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; AFP, alpha-fetoprotein; IL28B, interleukin 28B; ITPA, inosine triphosphate pyrophosphatase; OR, odds ratio.
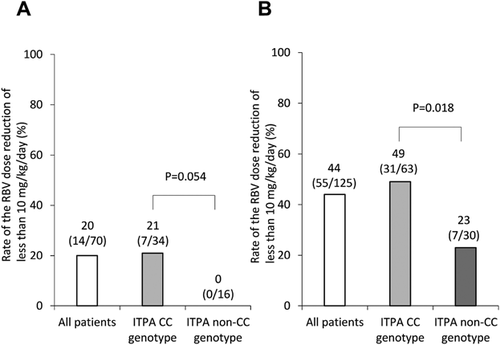
The Impact of Age and the ITPA Genotype on RBV Dose Reduction
Figure 3 shows the rate of the RBV dose reduction (RBV less than 10 mg/kg/day) according to age and the ITPA genotype. In patients less than 60 y.o., the RBV dose reduction (RBV less than 10 mg/kg/day) was 21% (7/34) for patients with the ITPA CC genotype and 0% (0/16) for those with the ITPA non-CC genotype, and the ITPA genotype was not significantly associated with the RBV dose reduction (P = 0.054) (Fig. 3A). On the other hand, in patients over 60 y.o., the RBV dose reduction (RBV less than 10 mg/kg/day) was 49% (31/63) for patients with the ITPA CC genotype and 23% (7/30) for those with the ITPA non-CC genotype, and the ITPA genotype was significantly associated with the RBV dose reduction (RBV less than 10 mg/kg/day) (P = 0.018) (Fig. 3B).
DISCUSSION
The antiviral therapy for CH-C has been changed greatly by the development of DAAs, which are divided into the combination therapy with IFN and DAAs and the combination therapy with multi-DAAs. In the combination therapy with multi-DAAs of sofosbuvir (NS5B polymerase inhibitor) and ledipasvir (NS5A inhibitor), a high SVR rate of more than 95% has been achieved without severe adverse effects [Afdhal et al., 2014a,2014b; Mizokami et al., 2015]. On the other hand, in SMV, Peg-IFN plus RBV combination therapy, high SVR rates of approximately 90% were attained for treatment-naïve patients [Hayashi et al., 2014; Izumi et al., 2014; Kumada et al., 2015]. Regarding the suppressive effects for carcinogenesis in SVR patients treated with IFN-based therapy, SMV, Peg-IFN plus RBV combination therapy is recommended as one of the IFN-based therapies for patients with the CH-C genotype 1 in Japan and Europe [Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology, 2014; European Association for the Study of the Liver, 2014]. Furthermore, SMV, Peg-IFN plus RBV combination therapy could be effective for some populations (patients with treatment emergent NS5A resistance associated variants, patients with renal impairment, patients receiving proton pump inhibitor treatment, etc.) whose SVR rates were decreased in sofosbuvir and ledipasvir therapy [Lawitz et al., 2015; Terrault et al., 2015]. However, patients with the IL28B minor genotype, non-response patients to previous treatment, and patients with advanced liver fibrosis have been reported to be difficult to treat [Forns et al., 2014; Hayashi et al., 2014; Izumi et al., 2014; Jacobson et al., 2014; Manns et al., 2014; Kumada et al., 2015]. Various adverse effects and long treatment durations (24 or 48 weeks) are also disadvantages of IFN-based therapy. Therefore, clinicians should have a good grasp on difficult to treat factors when deciding if they need to choose SMV, Peg-IFN plus RBV combination therapy. The present study demonstrated that the IL28B genotype and the RBV dose were independently associated with SVR, and the RBV dose affected the SVR rate dose-dependently. As for the RBV dose reduction of less than 10 mg/kg/day, the ITPA genotype and age were identified as significant factors.
In the present study, the IL28B genotype was significantly associated with SVR and was indicated to be a baseline predictor for treatment effect. The results of the present study were consistent with that of Peg-IFN plus RBV combination therapy and clinical trials of SMV, Peg-IFN plus RBV combination therapy [Ge et al., 2009; Tanaka et al., 2009; Hayashi et al., 2014; Jacobson et al., 2014; Manns et al., 2014]. Patients with the IL28B TT genotype achieved 95% SVR rate and were good indications for SMV, Peg-IFN plus RBV combination therapy.
In the present study, the impact of drug factors on treatment effect was examined in the view of devising an administration method that lead to improvement in the treatment effect of SMV, Peg-IFN plus RBV combination therapy. The RBV dose has been revealed to raise the SVR rate of Peg-IFN plus RBV combination therapy dose-dependently. As for the relationship between the RBV dose and the virologic response in Peg-IFN plus RBV combination therapy, patients who were administered ≥80% of planned doses of RBV were reported to achieve higher SVR rates than those administered <80% of planned doses of RBV [McHutchison et al., 2002]. Another study reported that the step-wise reduction of RBV dose was associated with a step-wise increase of the post-treatment relapse rate from 11% to 60% [Hiramatsu et al., 2009]. The present study showed that the RBV dose still played an important role on treatment effect even in SMV, Peg-IFN plus RBV combination therapy. In patients with the IL28B TT genotype, the SVR rate was high regardless of the RBV dose, whereas in patients with the IL28B non-TT genotype, the RBV dose affected the SVR dose-dependently. Especially, even in difficult-to-treat patients with the IL28B non-TT genotype, it revealed that patients administered ≥12 mg/kg/day of RBV achieved a 100% SVR rate. In patients with the IL28B non-TT genotype who were administered <10 mg/kg/day of RBV, the SVR rate was very low (44%). Keeping the RBV dose during treatment is very important to raise the SVR rate in patients with the IL28B non-TT genotype.
Next, because the baseline RBV dose is 10 mg/kg/day or more in the Japanese treatment protocol, the factors associated with a RBV dose reduction (RBV less than 10 mg/kg/day during treatment), except for 13 patients, whose RBV dose was reduced at the start, were examined. The ITPA genotype and age were selected as significant factors. The ITPA genotype was reported to be associated with hemolysis caused by RBV in Peg-IFN plus RBV combination therapy [Fellay et al., 2010; Ochi et al., 2010; Thompson et al., 2010], and the Hb decrease was more severe in patients with the ITPA major genotype than in those with the ITPA minor genotype. In SMV, Peg-IFN plus RBV combination therapy, it was reported that the Hb decrease was more severe in patients with the ITPA major genotype than in those with the ITPA minor genotype during SMV administration [Tahata et al., 2016]. The main reason for the RBV dose reduction was hemolytic anemia caused by RBV. Patients with the ITPA major genotype were more likely to develop hemolytic anemia caused by RBV and a more severe Hb decrease was observed compared to those with the ITPA minor genotype. In fact, the rates of the RBV dose reduction of less than 10 mg/kg/day according to the ITPA genotype were 15% (7/46) in patients with the ITPA non-CC genotype and 39% (38/97) for patients with the ITPA CC genotype (data are not shown). The rates of the RBV dose reduction of less than 10 mg/kg/day were significantly higher in patients with the ITPA CC genotype than in those with the ITPA non-CC genotype (P = 0.004).
As for the association between age and the RBV dose in IFN plus RBV combination therapy, the discontinuous rate of RBV was reported to be significantly higher in patients over 60 y.o. than in those less than 60 y.o. (21% vs. 9%, P = 0.0003), and the main reason for the RBV dose reduction was severe anemia in patients over 60 y.o. [Oze et al., 2006]. In Peg-IFN plus RBV combination therapy, the mean dose of RBV was shown to decrease with age (<55 y.o., 10.3 mg/kg/day; 55–59 y.o., 9.8 mg/kg/day; 60–64 y.o., 9.3 mg/kg/day; 65–69 y.o., 9.2 mg/kg/day; ≥70 y.o., 8.5 mg/kg/day) [Oze et al., 2011]. The main cause of this was considered to be the decrease in renal function with age and following the increase in serum RBV concentration in elderly patients. Patients less than 60 y.o. or patients with the ITPA non-CC genotype are expected to achieve a high SVR rate and are good candidates for SMV, Peg-IFN plus RBV combination therapy.
In conclusion, the IL28B genotype and the RBV dose are associated with SVR, and the RBV dosage affects the SVR rate dose-dependently in CH-C genotype 1 patients with the IL28B non-TT genotype treated with SMV, Peg-IFN plus RBV combination therapy. The ITPA genotype and age are associated with a RBV dose reduction of less than 10 mg/kg/day. SMV, Peg-IFN plus RBV combination therapy can be an option for the IL28B TT genotype patients and the IL28B non-TT genotype patients less than 60 y.o. or with the ITPA non-CC genotype.
ACKNOWLEDGMENTS
Other institutions and participants in the Osaka Liver Forum include the following: Osaka General Medical Center, A. Inoue; National Hospital Organization Minami Wakayama Medical Center, I. Yabuuchi; National Hospital Organization Osaka Minami Medical Center, T. Hijioka; Yao Municipal Hospital, H. Fukui; Saiseikai Senri Hosptial, K. Suzuki; Hyogo Prefectural Nishinomiya Hospital, Y. Inui; Otemae Hospital, Y. Doi; Sumitomo Hospital, A. Yamada; Kinki Central Hospital of Mutual Aid Association of Public School Teachers, M. Yamamoto; Higashi Osaka General Hospital, H. Matsumoto; Itami city Hospital, H. Aketa; Suita Municipal Hospital, T. Nagase; Ashiya Municipal Hospital, A. Takeda; Nishinomiya Municipal Central Hospital, H. Ogawa; Meiwa Hospital, Y. Hayakawa; Saso Hospital, M. Nishiuchi; Kano General Hospital, S. Kubota and Osaka Kaisei Hospital, N. Imaizumi.




