Gene expression analysis during acute hepatitis C virus infection associates dendritic cell activation with viral clearance
Abstract
Viral clearance during acute hepatitis C virus (HCV) infection is associated with the induction of potent antiviral T-cell responses. Since dendritic cells (DC) are essential in the activation of primary T-cell responses, gene expression was analyzed in DC from patients during acute HCV infection. By using microarrays, gene expression was compared in resting and activated peripheral blood plasmacytoid (pDC) and myeloid (mDC) DC from acute HCV resolving patients (AR) and from patients who become chronically infected (ANR), as well as in healthy individuals (CTRL) and chronically-infected patients (CHR). For pDC, a high number of upregulated genes was found in AR patients, irrespective of DC stimulation. However, for mDC, most evident differences were detected after DC stimulation, again corresponding to upregulated genes in AR patients. Divergent behavior of ANR was also observed when analyzing DC from CTRL and CHR, with ANR patients clustering again apart from these groups. These differences corresponded to metabolism-associated genes and genes belonging to pathways relevant for DC activation and cytokine responses. Thus, upregulation of relevant genes in DC during acute HCV infection may determine viral clearance, suggesting that dysfunctional DC may be responsible for the lack of efficient T-cell responses which lead to chronic HCV infection. J. Med. Virol. 88:843–851, 2016. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Clearance of hepatitis C virus (HCV) after acute infection occurs in a minority of patients and is associated with vigorous antiviral T-cell responses, whereas antiviral immunity is absent or very poor in individuals progressing to chronic infection [Diepolder et al., 1995; Missale et al., 1996; Thimme et al., 2001]. Different mechanisms have been postulated to explain the lack of T-cell responses in these patients, including generation of escape mutants [Bowen and Walker, 2005; Dazert et al., 2009], dysfunctional T-cell responses [Wedemeyer et al., 2002; Penna et al., 2007], or upregulation of immunoregulatory mechanisms [Cabrera et al., 2004; Rushbrook et al., 2005]. An important factor modulating T-cells responses are antigen presenting cells such as dendritic cells (DC), responsible for priming naive T-cells [Banchereau et al., 2000]. Upon pathogen detection, DC become activated, upregulating molecules associated to antigen presentation, costimulation, and lymphocyte-modulating cytokines, leading thus to induction of T-cell immunity. Two main DC subsets have been described in peripheral blood, myeloid DC (mDC) and plasmacytoid DC (pDC) [MacDonald et al., 2002], differing in their pathogen receptors, antigen-presenting capability, and cytokines produced. Presentation of antigens in the absence of DC activation usually leads to non-productive T-cell responses. Thus, dysfunctional or poor DC activation occurring during infections caused by several pathogens has been described as an escape mechanism leading to inefficient T-cell responses [Kruse et al., 2000; Beck et al., 2003].
In the case of HCV, as a single-stranded RNA virus, several innate immunity receptors have been described for viral sensing, including TLR3, TLR7, and RIG [Takahashi et al., 2010; Stone et al., 2013; Zhang et al., 2013]. Since strong differences in T-cell responses reported during acute hepatitis C associate with infection outcome and due to the role that DC play in priming these T-cell responses, gene expression was analyzed in DC during the acute phase of infection. The study considered patients with acute HCV infection who subsequently cleared the virus (acute resolvers; AR) and those who evolved to chronic HCV infection (acute non-resolvers; ANR), including in the analyses mDC and pDC subsets, in both cases unstimulated and after TLR-ligand stimulation. Gene expression patterns observed in these individuals as well as features of the different DC populations depending on the clinical outcome are shown below.
PATIENTS AND METHODS
Patients and Controls
Six patients with acute HCV infection were included in this study: two of them resolved the infection (AR) whereas remaining four evolved to chronic hepatitis (ANR). Peripheral blood mononuclear cell (PBMC) samples were obtained during the acute phase (weeks 3–6 after diagnosis) and frozen for analyses to be carried out after HCV resolution or chronification. As controls, four healthy seronegative individuals (CTRL) and four patients with chronic HCV infection were also included, and their PBMC submitted to similar procedures. Demographic, clinical, and virological data are shown in Table I. Informed consent was obtained from individuals included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committees (Ethical Committee of the Azienda Ospedaliero-Universitaria of Parma, Comité de Ética para la Investigación-Universidad de Navarra and Ethics Committee of the Faculty of Medicine of Casablanca). Informed consent was obtained from the parent/guardian of the minor involved in the study (patient ANR4).
| Patient | Age | Gender | ALT | AST | Viral load (log) | HCV genotype |
|---|---|---|---|---|---|---|
| AR1 | 26 | M | 1128 | 597 | 3.3 | 1a |
| AR2 | 43 | F | 340 | 320 | 3.5 | 1b |
| ANR1 | 39 | M | 688 | 144 | 3.6 | 1a |
| ANR2 | 28 | M | 202 | 105 | 3.5 | 3a |
| ANR3 | 29 | M | 1580 | 947 | 5.7 | 1b |
| ANR4 | 17 | F | 41 | 67 | 5.7 | 1b |
| CHR1 | 50 | M | 37 | 27 | 6.2 | 1a |
| CHR2 | 63 | M | 43 | 39 | 5.7 | 1b |
| CHR3 | 63 | F | 30 | 28 | 6.2 | 1b |
| CHR4 | 50 | M | 48 | 31 | 6.1 | 1b |
DC Preparation and Stimulation
To purify pDC, thawed PBMC were incubated with anti-CD304 (BDCA-4/Neuropilin-1)-coated magnetic beads, followed by purification of mDC using anti-CD1c (BDCA-1) beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Enriched cells were further purified by flow cytometry (FACSAria; BD-Biosciences, Franklin Lakes, NJ) after staining with CD11c-APC, HLA-DR-PerCP-Cy5.5, Lin-FITC and CD123-PE antibodies (BD-Biosciences). Dead cells were excluded by using the Sytox Blue (Life Technologies, Carlsbad, CA) cell death marker and in the Lin-HLA-DR+ gate, pDC were defined as CD11c−CD123+, while mDC were defined as CD11c+CD123−. Next, cells were washed with RPMI-1640 medium with 10% FCS and antibiotics (complete medium) and resuspended in a final volume of 100 μl of complete medium. Each sample were split in two tubes, one was left un-stimulated while the other was stimulated for 2 hr at 37°C with 1 μg/ml poly(I:C) (Amersham, Barcelona, Spain) + 250 μg/ml DEAE-Dextran (Sigma-Aldrich, St. Louis, MO) for mDC or 5 μg/ml of Imiquimod (Invivogen, San Diego, CA) for pDC. Then cells were centrifuged, supernatant discarded, and pellets resuspended in Amplification Buffer (Miltenyi Biotec) before freezing them.
mRNA Amplification and Microarray Experiments
Since some samples contained less than 104 cells, the SuperAmp technology (Miltenyi Biotec) was used to amplify mRNA before transcriptomic analysis. Samples processed according to Miltenyi instructions were sent for mRNA amplification and subsequent analyses. Amplified cDNA samples were quantified, their integrity checked and processed using manufacturer protocols for hybridization to the Agilent SurePrint G3 Human Gene Expression 8 × 60 K v2 microarray.
Data Analysis
Microarray data normalization was performed using quantile algorithm for experiments in pDC and mDC. After quality assessment, a filtering process was carried out to eliminate low expression probe sets. Applying the criterion of an expression value greater than 32 in at least two samples of one of the experimental conditions (stimulated or non-stimulated ANR, AR, and CTRL), 38208 probe sets were selected for statistical analysis in the case of pDC cells and 38407 in mDC cells. LIMMA (Linear Models for Microarray Data) [Smyth, 2004] was used to identify the probe sets with significant differential expression between experimental conditions. Genes were selected as significant using a P-value cut off P < 0.001. Data processing and statistical analysis were performed with R and Bioconductor [Gentleman et al., 2005]. Enrichment analysis of biological processes, molecular functions, and Kegg pathways were carried out through the use of PANTHER and GATHER algorithms [Chang and Nevins, 2006; Mi et al., 2013]. The R function “pvclust” was used to conduct multiscale bootstrap resampling in hierarchical clustering [Suzuki and Shimodaira, 2006]. Microarray data were deposited in GEO under accession number GSE65123.
RESULTS
Differentially Expressed Genes in DC Between AR and ANR Patients
To analyze differences in DC between AR and ANR patients, six individuals with acute hepatitis C were recruited and followed up until disease resolution or establishment of chronic infection. Once clinical outcome was evident, pDC and mDC were highly purified from frozen samples obtained at 3–6 weeks after diagnosis of acute hepatitis C. Their purity was above 99%, and pDC and mDC were either unstimulated (NST) or treated (ST) for 2 hr with Imiquimod or poly(I:C), respectively.
In initial studies, 196 differentially expressed genes (DEG) were found in NST pDC from AR and ANR patients, whereas 50 DEG were observed for NST mDC (Fig. 1A,B and Supplementary data 1). None of these genes was common to pDC and mDC. In the case of ST cells, 139 genes were found for pDC, whereas the number of DEG increased to 185 for mDC (Fig. 1A,B and Supplementary data 1). Two common DEG for stimulated mDC and pDC were found. Regarding the sign of differences, most DEG corresponded to upregulated genes in AR, although downregulated genes in AR increased after stimulation (Fig. 1C).
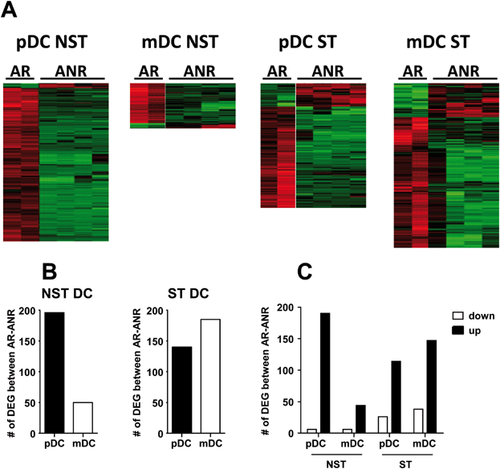
Analyses of Biological Processes, Molecular Functions and Pathways of DEG Between DC From AR and ANR Patients
To understand the significance of differential gene expression, biological processes related to these DEG were analyzed and it was found that, both for untreated and stimulated DC, genes associated to metabolic processes (Fig. 2) (mainly those corresponding to nucleobase-containing metabolic processes and protein translation, modification, and proteolysis) were statistically over-represented (P < 0.001), except in the group of NST mDC. Regarding molecular functions, many genes corresponded to catalytic and binding activity, with ligase and nucleic acid binding activities enriched in NST and ST pDC (P < 0.01) and nucleic acid binding activity-relate genes enriched in ST mDC (P < 0.001).
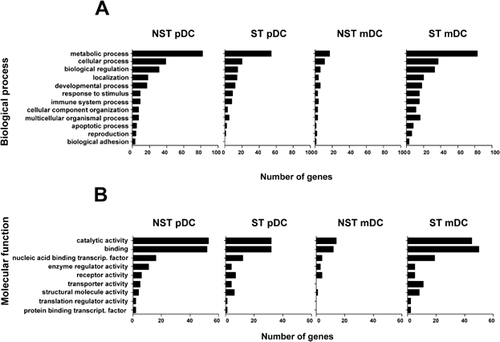
Kegg pathways analyses in the same groups of DEG showed that Ubiquitin-mediated proteolysis pathway (P = 0.001) and the Tricarboxylic acid cycle pathway (P < 0.01) were enriched in pDC, whereas genes belonging to Cytokine/cytokine receptor interaction pathway (P < 0.05), the Oxidative phosphorylation (P < 0.05) and MAP kinase (P < 0.05) pathways were enriched in mDC (Table II).
| AR/ANR comparison | |||
|---|---|---|---|
| Cells | Pathway | Genes | P |
| NST pDC | Ubiquitin-mediated proteolysis | ↓ANAPC1, ↓HERC1, ↓HERC2, ↓SMURF1** | P = 0.001 |
| ST pDC | TCA cycle | ↓PCK1, ↓SDHC | P = 0.008 |
| NST mDC | Cytokine/cytokine receptor | ↓CXCR5, ↓BMPR1A | P = 0.01 |
| ST mDC | Oxidative phosphorylation | ↓ATP6V0B, ↓NDUFAB1, ↓NDUFS6, ↓NDUFS7 | P = 0.03 |
| MAPK signaling | ↓FLNB, ↓IL1B, ↓MAP2K7, ↓MAPK6, ↓PAK1 | P = 0.04 | |
- * DEG obtained after comparing the different groups of DC between AR and ANR patients were analyzed by using the Gather software (http://gather.genome.duke.edu). Only those Kegg pathways with statistically significant enrichment are shown.
- ** Arrows indicate up- or down-regulated genes in ANR patients.
Although most DEG corresponded to upregulated genes in AR patients (as shown in Fig. 1C), downregulated genes were also studied separately. Enrichment analysis of these genes did not reveal any specific process, function, or pathways different from those observed when analyzing all DEG. These results suggest that the lower expression in ANR patients of pathways and individual genes relevant for DC functions such metabolism [Pearce and Everts, 2015], activation, antigen presentation [Moffat et al., 2013], or antiviral [Yoshio et al., 2013; Zhang et al., 2013] and inflammatory cytokine/chemokine and receptors (CSF1, IL28A, IL1B, IL-4R, IFNGR, CXCR5) and ligands/receptors modulating DC/T-cell activity (LILRB2, MICB, AGER) may be responsible for the inefficient antiviral immunity of these individuals.
Comparison of DEG Between AR and ANR With Control Groups
To study the relevance of DEG found in patients with acute hepatitis C, their expression was also analyzed in healthy individuals (CTRL). By using the R function “pvclust” to conduct multiscale bootstrap resampling in hierarchical clustering, it was observed that, based on the expression of these DEG, samples from CTRL clustered together with AR in both NST and ST pDC (AR-CTRL vs. ANR P < 0.05) (Fig. 3A). A similar clustering was found in ST mDC where, with the exception of an ANR patient, remaining ANR individuals clustered apart from AR and CTRL (AR-CTRL vs. ANR P < 0.05) (Fig. 3B).
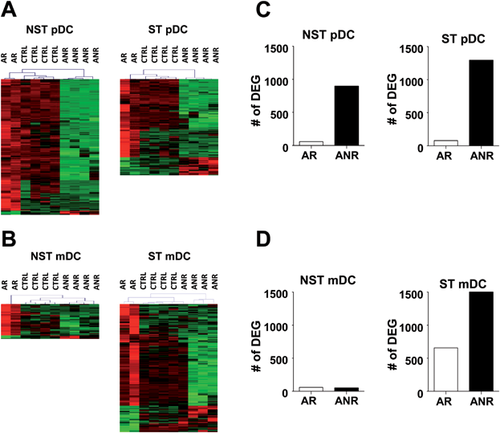
Moreover, to have a broader insight, comparison of all genes was also considered, using the same criteria as in AR/ANR comparisons (Supplementary data 2). In the case of pDC, irrespective of stimulation, the number of DEG was clearly higher when comparing DC from ANR with CTRL than in AR/CTRL comparisons (Fig. 3C), suggesting important differences between ANR and AR or CTRL. When analyzing mDC, unstimulated mDC from both AR and ANR had a low number of DEG in comparisons with CTRL. However, stimulation of mDC increased the number of DEG, although differences were again higher between CTRL and ANR than between CTRL and AR (Fig. 3D). These new results, which also include the study of healthy subjects, not only resemble initial differences observed in direct AR/ANR comparisons, but also highlight the divergent behavior of DC from ANR patients in comparison with healthy individuals.
Pathway analyses were also carried out for DEG found in comparisons between AR/ANR patients and CTRL group (Table III). No pathways with significant enrichment were found in any CTRL/AR comparison. By contrast, in CTRL/ANR analyses, both in NST and ST pDC, genes corresponding to pathways such as Histidine metabolism (P < 0.01) as well as Tight junction, Gap junction, Focal adhesion, JAK/STAT, Insulin signalling, and Wnt signalling (P < 0.05 in all pathways) were observed. In the case of mDC, as previously observed in direct AR/ANR comparisons, enriched pathways were mainly evident after stimulation. Thus, although only the Cytokine/cytokine receptor pathway (P < 0.001) was enriched in NST mDC, Apoptosis (P < 0.05), Focal adhesion (P < 0.05), Toll-like receptor (P < 0.01), MAP kinase (P < 0.01), and Insulin signalling (P < 0.01) pathways were found in ANR/CTRL comparisons in ST mDC. Finally, as ocurred in AR/ANR comparisons, in most cases, differences corresponded to DEG which were down-regulated in ANR patients with respect CTRL group.
| CTRL/ANR comparison | |||
|---|---|---|---|
| Cells | Pathway | Genes | P |
| NST pDC | Tight junction | ↑CLDN7 ↓CSNK2A2 ↓EPB41L1 ↓KRAS2 ↓PPP2CB ↓PRKCB1 ↓PRKCH ↓RAB13** | P = 0.02 |
| Gap junction | ↓ADCY3 ↑DRD2 ↑GRM6 ↑GUCY1B3 ↓KRAS ↓MAP3K2 ↓PRKCB1 ↓RAF1 | P = 0.04 | |
| Focal adhesion | ↑COL1A1 ↓CAPN2 ↓FARP2 ↑FGR ↑IGF1 ↓KRAS2 ↓PRKCB1 ↓PXN ↓RAF1 ↓SHC2 ↑THBS4 | P = 0.01 | |
| Histidine metabolism | ↓AOC3 ↓HARS ↑HDC ↓PRMT6 ↓LCMT2 ↓SH3GLB1 ↑WBSCR22 | P = 0.02 | |
| ST pDC | Insulin signalling | ↑FBP1 ↓KRAS ↓MAP2K2 ↓MAPK6 ↑PCK1 ↓PDPK1 ↓PIK3CA ↑PIK3R2 ↓PPP1CC ↓PRKAB1 ↓PRKAR1A ↓RHOQ ↓SHC1 | P = 0.02 |
| Wnt signalling | ↓CSNK2A1 ↓CSNK2A2 ↓FBXW11 ↑PPP3R2 ↓PRKCB1 ↑SFRP1 ↑WNT5B | P = 0.03 | |
| Jak/STAT | ↓JAK1 ↓EPO ↑IL2RB ↓IL28RA ↓IL4R ↓IL6ST ↓PIK3CA ↑PIK3R2 ↓PIAS1 ↓PTPN11 ↓SPRY1 ↓CNTF | P = 0.04 | |
| Focal adhesion | ↓CAPN2 ↓CDC42 ↑CHAD ↑COL5A1 ↑IGF1 ↓ITGB1 ↓KRAS2 ↓PPP1CC ↑LAMA5 ↑LAMB1 ↓PDPK1 ↑PIK3R2 ↓PRKCB1 ↓SHC1 | P = 0.03 | |
| NST mDC | Cytokine/cytokine receptor | ↓CRLF2 ↓IL7R ↓IL8 ↓TGFB2 ↓TNFSF14 | P = 0.0002 |
| ST mDC | MAPK signalling | ↓AKT2 ↓ARRB2 ↑BRAF ↓CASP8 ↓DUSP10 ↓DUSP4 ↓HSPA8 ↓IKBKB ↓IL1B ↓MAPK8 ↓PPM1B ↑PPP3R2 ↓PRKCG ↓RAC1 ↓RAF1 ↓SOS2 ↓TP53 | P = 0.005 |
| Insulin signalling | ↓AKT2 ↓EIF4E2 ↑EIF4EBP1 ↓IKBKB ↓INPPL1 ↓MAPK8 ↓PFKP ↓PHKB ↓PIK3CB ↓PRKAA1 ↓PRKAG2 ↓RAF1 ↓RAPGEF1 ↓RHOQ ↓RPS6 ↓SOCS3 ↓SOS2 ↓TRIP10 | P = 0.007 | |
| Toll-like receptor signalling | ↓AKT2 ↓CASP8 ↓CCL4 ↓CXCL10 ↓IFNB1 ↓IKBKB ↓IL1B ↓IL8 ↓MAPK8 ↓NFKBIA ↓PIK3CB ↓RAC1 ↓STAT1 ↓TLR5 | P = 0.008 | |
| Focal adhesion | ↓AKT2 ↓BRAF ↓MAPK8 ↓PIK3CB ↓PPP1R12A ↓PRKCG ↓RAC1 ↓RAF1 ↓ROCK1 ↓SOS2 ↓SPP1 ↓SRC ↓TESK2 ↓VEGFA ↓VEGFB ↓VTN | P = 0.02 | |
| Apoptosis | ↓AKT2 ↓CASP8 ↓CFLAR ↓IKBKB ↓IL1B ↓NFKBIA ↓PIK3CB ↑PPP3R2 ↑TP53 | P = 0.03 | |
- * DEG obtained after comparing DC from ANR patients and CTRL were analyzed by using the Gather software (http://gather.genome.duke.edu). Only those Kegg pathways with statistically significant enrichment are shown.
- ** Arrows indicate up- or down-regulated genes in ANR patients.
Finally, in order to study if these differences were only observed in comparisons ANR versus AR or CTRL, we expanded the analysis of expression relevant DEG found in patients with acute HCV infection to a group of individuals with chronic HCV infection (CHR). We found that for most DC (NST and ST pDC, as well as for ST mDC), ANR not only clustered apart from AR and CTRL, but also from CHR patients (ANR vs. AR-CTRL-CHR. P < 0.05) (Supplementary Data 3). Moreover, when considering all genes, as occurred when using the CTRL group, ANR had higher differences with CHR than AR, confirming the idea of a distinct pattern in ANR patients from remaining groups.
DISCUSSION
The cellular immune response associated to different clinical outcomes after acute hepatitis C suggests that this response may be a fundamental factor determining viral clearance. Since initial T-cell responses are primed by DC, in the present study, gene expression by the two main peripheral blood DC subsets were investigated in patients with acute hepatitis C, both in basal conditions and after stimulation with TLR ligands potentially related to those displayed by HCV. We acknowledge the limitations of our study due to the low number of patients included (two AR, four ANR, and CHR patients), as well as potential variation in clinical/virological status and age. However, despite the lack of longitudinal studies and validation of individual findings which would have shed more light on the relevance of identified genes, we believe that differences observed may globally reflect the status of these DC subsets in individuals with acute hepatitis C.
Analysis of DEG between AR and ANR patients shows that unstimulated pDC and mDC have a distinct pattern. Thus, without considering the nature of genes involved, it is interesting to note that before stimulation, the number of DEG in pDC is higher than in mDC. This is observed not only in direct AR/ANR comparisons, but also when including in the analysis CTRL as well as CHR individuals. Indeed, for pDC, AR patients have less DEG (about 100) than ANR (500–1,000), whereas for mDC, both AR and ANR patients have few differences with control groups. This lack of differences in unstimulated mDC irrespective of clinical outcome is in agreement with data reporting that mDC in patients with acute HCV infection have an immature phenotype, which is observed both in AR and ANR [Pelletier et al., 2013].
Besides differences in the type of DC, another interesting feature is the pattern observed between pDC and mDC in terms of gene expression considering unstimulated versus stimulated DC. Thus, although for pDC, there are not considerable differences in DEG number between NST and ST cells, these differences clearly increase in mDC after stimulation, something which is again observed when including in the analysis DC from CTRL and CHR. Indeed, for pDC, the main differential factor is the type of patients, irrespective of stimulation, while for mDC, the main differential factor is cell stimulation. These results showing important upregulation of genes in pDC from AR patients during acute HCV infection, suggest that ANR patients do not sufficiently express genes potentially implicated in mechanisms responsible for viral clearance. Since this occurs irrespective of stimulation status it may imply that pDC derangement is a general phenomenon in ANR patients.
Metabolic processes are well represented by DEG observed in pDC, related to ligase and nucleic acid binding activities. This is evident for the Ubiquitin-mediated proteolysis pathway, which in the case of DC may be related to regulation of antigen presentation to T-cells at different levels, including processing of antigenic substrates, upregulation of MHC-II molecules or its degradation [Moffat et al., 2013], as well to the Tricarboxylic acid pathway, potentially related to the generation of cell constituents necessary for DC activation [Everts et al., 2014]. Indeed, the importance of metabolism on DC, which is significantly enriched in AR patients, has become evident during the last years [Everts and Pearce, 2014]. Thus, TLR activation induces metabolic switch towards a new profile relying on aerobic glycolysis [Krawczyk et al., 2010], useful to provide precursors and constituents needed for the functions which should be displayed by mature DC. Regarding this pathway, it is interesting to note that HK1, encoding enzyme Hexokinase 1, is up-regulated in NST pDC in AR. Hexokinase 1 is the first enzyme of the glycolytic pathway, has been described as one of its key control elements and is also an antiapoptotic molecule [Gottlob et al., 2001]. In DC, hexokinase activity increases in activated DC after stimulation [Everts et al., 2014]. Thus, our results suggest that DC from AR display a more active metabolism useful for DC functions.
In the case of mDC, few differences are observed between AR and ANR patients in unstimulated cells, even when considering comparisons with CTRL or CHR. However, these differences increase after stimulation, suggesting that only upon pathogen sensing their differential properties become evident. Interference with DC activation pathways is a common strategy used by pathogens to avoid antigen presentation and activation of protective T-cell responses [Kruse et al., 2000; Beck et al., 2003]. In the case of HCV, although viral infection of DC does not seem to be a common event [Marukian et al., 2008], we demonstrated that structural proteins inhibited TNF-alpha- and CD40L-dependent maturation and concomitant induction of antiviral T-cell responses [Sarobe et al., 2003], without any effect on LPS-induced DC activation. Thus, viral proteins may interfere with signalling pathways triggered by specific ligands, while leaving unaffected other pathways. Alternatively, even in the absence of DC infection, it has been shown that extracellular viral proteins [Dolganiuc et al., 2003; Nattermann et al., 2006] or cytokines induced by these proteins [Dolganiuc et al., 2006] may modulate DC responsiveness, leading to abnormal activation. Analysis of biological processes and functions corresponding to differentially expressed genes shows that different gene sets are represented. Besides pathways corresponding to Oxidative phosphorylation and MAPK signalling, genes directly related to immune and antiviral functions, such as those encoding cytokines (CSF1, IL28A, IL1B), cytokine and chemokine receptors (IL-4R, IFNGR, CXCR5) and ligands/receptors modulating DC/T-cell activity (LILRB2, MICB, AGER) are differentially expressed. Up-regulated production in AR of inflammatory cytokines like IL-6, IL-12, and TNF-α, as well as association with antiviral responses has been already reported [Pelletier et al., 2013], reinforcing their role in viral clearance during acute HCV infection. Moreover, a gene of particular interest up-regulated in ST mDC from AR patients is IL28A. This gene, encoding IFN-λ2, belongs to the group of type III IFNs, a family of cytokines which has gained much attention during the last years due to the association between polymorphisms related to IL28B and viral clearance after acute hepatitis C [Thomas et al., 2009] or treatment [Ge et al., 2009]. These cytokines possess clear anti-HCV properties [Diegelmann et al., 2010] and their production in response to HCV is mainly dependent on BDCA-3+ mDC [Yoshio et al., 2013; Zhang et al., 2013]. Thus, up-regulated expression in AR of cytokines and receptors associated to inflammatory and antiviral properties would suggest that these factors may be associated with viral clearance after acute HCV infection. In a similar manner, HIF1A, a transcription factor involved in promoting the glycolytic pathway and associated with myeloid cells and their effector functions [Cramer et al., 2003], has upregulated expression in ST mDC from AR. These examples stress the presence of a metabolic profile related to an activated DC in AR patients, suggesting that improper activation of key metabolic pathways may be related to the lack of efficient DC functions.
Comparisons between AR/ANR patients and CTRL group reinforce results obtained in direct AR/ANR comparisons. In general, gene expression profile of ANR patients is divergent from that of remaining groups. The lower expression in ANR of genes associated to pathways like Cytokine/cytokine receptors, Toll-like receptor, MAPK, Insulin, Jak/STAT, and Wnt signalling pathways, which play an important role in DC, may be related to poorer of DC activation in these individuals, with a concomitant decreased functional capacity. Interestingly, the same divergent behavior of ANR patients is observed when considering CHR individuals, which cluster closer to remaining groups, suggesting that the poorer DC functions observed in ANR is a feature of these patients, mainly observed during the acute phase but not at later stages during viral chronification.
Finally, it has to be noted that our analysis refers to peripheral blood DC, which may not completely reflect behavior of intrahepatic DC. Indeed, enhanced intrahepatic DC frequency, as occurs with CD141+ mDC [Kelly et al., 2014], and their activated phenotype [Velazquez et al., 2012] are factors not considered in this study, which may modulate viral clearance during acute HCV infection.
In summary, DC from patients with acute HCV infection display different gene expression patterns according to viral clearance. DC population (pDC vs. mDC) and stimulation status are important factors implicated in differences between patients eliminating HCV and those who become chronically infected. Upregulation of genes involved in metabolic processes as well as in different DC activation pathways may be responsible for the stronger immune responses observed in patients clearing HCV, suggesting that factors modulating DC activity may play an important role in viral clearance during acute HCV infection.
ACKNOWLEDGMENTS
Authors would like to thank E. Santamaria and V. Segura for their help in data analysis and V. Villar for sample handling.




