Measles surveillance in Taiwan, 2012–2014: Changing epidemiology, immune response, and circulating genotypes
Abstract
In Taiwan, although the coverage rate of two doses of measles-containing vaccine has been maintained at over 95% since 2001, measles outbreaks occurred in 2002, 2009, and 2011. The present study reports that 43 cases were confirmed by laboratory testing in Taiwan in 2012–2014 and that adults have emerged as one of groups susceptible to measles virus (MV) infection, who may have discrepant humoral immune reactions—indicated by the level of IgM and IgG antibodies compared to a naïve, susceptible measles case. Thirty-seven of 43 cases confirmed by RT-PCR were further characterized by genotyping. In Taiwan, genotype H1 was the major strain in circulation prior to 2010, while D9 was the most frequently detected MV genotype between 2010 and 2011. The genotyping data collected between 2012 and 2014 revealed that H1 rebounded in 2012 after an absence in 2011 and was imported from China and Vietnam. In 2014, genotype B3 first appeared in Taiwan following import from the Philippines and became the most frequently detected strain. Genotype D8, linked to importation from various countries, including India, Indonesia, Thailand, and Vietnam, showed sequence divergence. D9 was imported from Malaysia in 2014. The MV genotypes detected in Taiwan reflected the genotypes of circulating endemic measles strains in neighboring countries. A significant rise in the number of measles cases and in measles with genotypes imported from surrounding countries indicated that measles resurged in Asia in 2014. J. Med. Virol. 88:746–753, 2016. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Measles is a highly contagious disease caused by the measles virus (MV), a member of the genus Morbillivirus of the Paramyxoviridae family, but can be controlled through administration of the measles vaccine [Durrheim et al., 2014]. A policy mandating routine vaccination was implemented in Taiwan in 1978 and coverage of over 95% for primary and secondary dose vaccines containing MV has been maintained since 2001 [Liu et al., 2014]. Nevertheless, measles outbreaks occurred in highly immunized communities in 2002, 2009, and 2011 [Cheng et al., 2013]; measles infections in vaccinated individuals result in mild symptoms and discrepant laboratory characteristics compared to those in unvaccinated individuals [Atrasheuskaya et al., 2008; Coleman and Markey, 2010; Hickman et al., 2011; Rosen et al., 2014]. Interestingly, a high proportion of measles cases among young adults has been seen in countries with high immunization rates, including Australia, Switzerland, and Taiwan [Jayamaha et al., 2012; Cheng et al., 2013; Delaporte et al., 2013]. In 2014, measles rebounded in many countries in the Western Pacific Region [Takahashi et al., 2014; Yang et al., 2015], especially in the Philippines, China, and Vietnam [Butler, 2015]. In the USA, more than 600 cases were reported [McCarthy, 2015], surpassing the highest reported yearly total of measles cases (220 cases in 2011) since its elimination [Rosen et al., 2014].
Molecular epidemiology is a useful tool for tracking the transmission routes of MV [Rota et al., 2011] and analysis of shifts in the prevalence of MV strains over time could help document the success of efforts to eliminate measles and an understanding of whether an endemic strain is extinct or reseeded across the world. Thus, laboratory surveillance of MV provides continuing scrutiny and plays an important role in the process of measles elimination. In this study, it was found that the genotype B3 MV was newly imported into Taiwan in 2014 and the molecular epidemiology of dynamic changes in MV genotypes in Taiwan was characterized. The data indicate that circulating measles strains are continually imported from surrounding endemic countries, making the goal of eliminating measles difficult and posing a challenge to countries already declared free of the virus.
MATERIALS AND METHODS
Collection of Clinical Samples
Patients whose fever reached 38°C, accompanied by a rash and one of the three of the following features qualified as suspected measles cases and were reported to the Taiwan Centers for Disease Control (Taiwan CDC): (1) cough, coryza, or conjunctivitis, (2) not immunized with a measles-containing vaccine, or (3) had travelled abroad to a region with endemic measles within 3 weeks of admission. Blood, throat swabs, and urine were collected to confirm the presence of the virus.
Ethics Statement
Measles is a notifiable disease in Taiwan, and clinical specimens of suspected cases are required to be collected and tested for the measles virus according to the Communicable Disease Control Act. This study did not involve any activities that were reviewed prospectively by an ethics committee, and informed consent from suspected cases was exempted. This study was approved for publication by the Taiwan CDC.
Antibody Detection, Virus Isolation, and Viral RNA Detection
MV-specific IgM and IgG antibodies were identified in serum using an enzyme-linked immunosorbent assay (ELISA) with a commercially available kit (Siemens, Marburg, Germany), according to the manufacturer's instructions. The IgM/IgG index ratios were calculated by dividing the net absorbance values measured for IgM by those for IgG. An IgM/IgG ratio >1 represented a primary immune response to measles, and ratios <1 were consistent with a secondary response [Rosen et al., 2014].
Clinical throat swab and/or urine specimens were inoculated onto B95a cells, a marmoset B lymphoblastoid cell line transformed by Epstein–Barr virus, and observed for the presence of a cytopathic effect (CPE). Inoculated cells were blind passaged up to two times and those showing no evidence of CPE were discarded.
Total RNA was extracted from measles isolates or directly from clinical specimens collected by throat swab or urine with a MagNa Pure Compact Nucleic Acid Isolation kit I (Roche, Mannheim, Germany) according to the manufacturer's instructions. Partial nucleoprotein segments (the C-terminus of the N gene) of MV were amplified by RT-PCR with the primers Me214 and Me216 and a one-step RT-PCR kit (Qiagen, Hilden City, Germany), as previously described [Bankamp et al., 2013]. The H gene of MV was amplified and sequenced using RT-PCR with the primers H1F, H2R, H3F, and H4R (Supplementary Table I) and a one-step RT-PCR kit (Qiagen).
Sequencing, Genotyping, and Phylogenetic Analysis
Sequencing reactions were performed with an ABI BigDye Terminator V3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. MV genotypes were determined by phylogenetic analysis of the 456-nt sequences of the viral N gene, together with WHO-designated reference sequences for each genotype [WHO, 2012]. A phylogenetic tree was constructed with MEGA software version 4.0 [Tamura et al., 2007] based on neighbor-joining methods, using 1,000 bootstrap replicates. The sequences generated in this work are available under the following NCBI accession numbers: partial N gene – KJ437137 to KJ 437152, KJ834003 to KJ 834011, KP769788 to KP769799; H gene – KP881499 to KP881504.
RESULTS
A total of 351 suspected measles cases were reported in Taiwan between 2012 and 2014 and, for all of them, at least one serum and throat swab or urine sample were tested in the laboratory. Forty-three cases were confirmed by laboratory testing for the presence of MV immunoglobulin M (IgM) and/or viral RNA (Table I). Of these, 26 cases (60.5%) were male (Table II) and 10 were children (23.3%) younger than 12 months old, who were not eligible for immunization. In addition, there were four children (9.3 %) between 12 and 24 months, one of whom had been immunized with a single dose of measles, mumps, and rubella (MMR) vaccine, while the others had not been immunized (Table II). Adults accounted for 29 cases (67.4%), ranging in age from 19 to 45 years (birth cohort 1969–1995). Five of the 29 adults had not received MV vaccines, 6 had been vaccinated, and 18 reported an unknown vaccination history. The serological data indicated that the percentages of cases with an IgM/IgG index ratio <1, which is associated with a secondary immune response, in the adult groups (19–36 years: 68% [15/22]; 37–45 years: 71% [5/7]) were higher than in the child groups (aged ≤2 years: 21% [3/14]; Table II). The period (days) between the onset of rash and serum sampling is shown in Table I. The periods between cases with IgM/IgG index ratios of <1 and >1 did not differ significantly (4.35 ± 4.57 vs. 4.75 ± 4.53 [Ave ± sd], one-tailed t-test: 0.39). These results agree with previous vaccination data, indicating that most children aged ≤2 years have not yet received the MV vaccine (93%, 13/14) and are, therefore, presumed to be non-immune and susceptible (Table II).
| Year | Case no. | Age (yy/mm) | Vaccine history | Genotype | Strain designation | IgM result | IgM/IgG ratioa | Interval (days)b | Imported countryc |
|---|---|---|---|---|---|---|---|---|---|
| 2012 | 1 | 21/03 | Unknown | D8 | MVs/Taipei.TWN/10.12 | P | 2.30 | 6 | IND |
| 2 | 29/09 | MV | H1 | MVs/Taoyuan.TWN/11.12 | E | 0.09 | 1 | CHN | |
| 3 | 23/03 | Unknown | D8 | MVi/Tainan.TWN/12.12 | P | 88.0 | 2 | THA | |
| 4 | 01/02 | No | –d | – | P | 1.35 | 18 | CHN | |
| 5 | 24/03 | Unknown | H1 | MVs/Keelung.TWN/17.12 | P | 0.17 | 3 | CHN | |
| 6 | 27/09 | No | H1 | MVs/Taipei.TWN/22.12 | P | 28.5 | 5 | Dom | |
| 7 | 28/08 | Unknown | H1 | MVs/Taichung.TWN/23.12 | P | 0.18 | 6 | Dom | |
| 8 | 27/05 | MV | H1 | MVi/Taipei.TWN/24.12 | N | 0.02 | 0 | Dom | |
| 9 | 24/09 | Unknown | D8 | MVi/Taipei.TWN/36.12 | P | 28.1 | 7 | Dom | |
| 2013 | 10 | 00/08 | No | H1 | MVs/Kaohsiung.TWN/06.13 | P | 142.3 | 2 | CHN |
| 11 | 00/10 | No | H1 | MVs/Kaohsiung.TWN/07.13 | P | 1.78 | 16 | CHN | |
| 12 | 37/11 | Unknown | – | – | P | 0.233 | 1 | KOR | |
| 13 | 00/10 | No | H1 | MVs/Taoyuan.TWN/16.13 | N | 0.66 | 3 | CHN | |
| 14 | 28/01 | MMR | D8 | MVs/Taipei.TWN/31.13/1 | E | 0.12 | 7 | Domestic | |
| 15 | 25/06 | Unknown | D8 | MVi/Taipei.TWN/31.13/2 | N | 0.56 | 2 | Domestic | |
| 16 | 44/04 | Unknown | H1 | MVi/Taipei.TWN/33.13/1 | P | 5.56 | 6 | CHN | |
| 17 | 39/07 | MMR | H1 | MVi/Taichung.TWN/33.13/2 | N | 0.09 | 5 | CHN | |
| 2014 | 18 | 28/05 | Unknown | B3 | MVs/Kaohsiung.TWN/02.14 | P | 0.08 | 1 | PHL |
| 19 | 00/07 | No | B3 | MVs/Hsinchu.TWN/06.14 | P | 138.5 | 4 | PHL | |
| 20 | 00/10 | No | B3 | MVi/Kaohsiung.TWN/07.14 | P | 119.8 | 4 | PHL | |
| 21 | 00/10 | No | – | – | P | 0.66 | 18 | PHL | |
| 22 | 00/09 | No | D8 | MVi/Taipei.TWN/11.14 | P | 159.7 | 3 | IDN | |
| 23 | 01/00 | No | D9 | MVi/Hualien.TWN/12.14 | E | 84.5 | 1 | MYS | |
| 24 | 29/08 | Unknown | H1 | MVs/Taipei.TWN/14.14 | E | 0.06 | 1 | CHN | |
| 25 | 39/08 | No | – | – | P | 0.10 | 4 | CHN | |
| 26 | 24/02 | Unknown | B3 | MVi/Taichung.TWN/16.14 | P | 0.19 | 5 | PHL | |
| 27 | 20/11 | MMR | B3 | MVs/Taichung.TWN/17.14/1 | N | 0.05 | 1 | PHL-R | |
| 28 | 22/01 | Unknown | B3 | MVs/Taichung.TWN/17.14/2 | N | 0.04 | 1 | PHL-R | |
| 29 | 01/02 | No | D8 | MVi/Pingtung.TWN/18.14 | P | 527 | 3 | VNM | |
| 30 | 45/09 | No | H1 | MVi/Taipei.TWN/22.14 | P | 3.51 | 4 | CHN | |
| 31 | 40/10 | No | – | – | P | 0.13 | 10 | CHN | |
| 32 | 38/01 | Unknown | – | – | P | 0.08 | 15 | CHN | |
| 33 | 24/01 | No | H1 | MVs/Taipei.TWN/23.14 | N | 0.06 | 3 | CHN-R | |
| 34 | 28/11 | Unknown | D8 | MVs/Taoyuan.TWN/25.14 | E | 0.06 | 4 | HK | |
| 35 | 02/00 | No | D8 | MVs/Taoyuan.TWN/33.14 | P | 91 | 2 | IDN | |
| 36 | 24/06 | Unknown | B3 | MVi/Taichung.TWN/36.14/1 | P | 11.7 | 1 | PHL | |
| 37 | 01/00 | No | H1 | MVi/Taoyuan.TWN/36.14/2 | P | 349 | 4 | CHN | |
| 38 | 24/07 | Unknown | B3 | MVi/Nantou.TWN/42.14 | E | 0.32 | 6 | Dom | |
| 39 | 27/00 | Unknown | B3 | MVi/Nantou.TWN/43.14 | E | 5.6 | 1 | Dom-R | |
| 40 | 26/06 | Unknown | B3 | MVi/Nantou.TWN/44.14/1 | E | 5.0 | 3 | Dom-R | |
| 41 | 23/08 | MMR | B3 | MVs/Tainan.TWN/44.14/2 | E | 0.02 | 2 | Dom-R | |
| 42 | 01/06 | MMR | B3 | MVs/Nantou.TWN/44.14/3 | N | 0.06 | 1 | Dom-R | |
| 43 | 00/10 | No | H1 | MVs/Taipei.TWN/44.14/4 | P | 60.5 | 3 | VNM |
- MV, measles vaccine; MMR, measles-mumps-rubella vaccine; P, positive; E, equivocal; N, negative.
- a IgM/IgG ratio calculated from raw OD of ELISA in serum. IgM/IgG ratio >1 suggests a primary immune response to measles and ratio <1 suggests a secondary response.
- b Indicate interval between date of rash onset and serum sampling.
- c Imported country refers to any country to which a patient travelled 7–21 days before the onset of the rash. Countries are abbreviated according to ISO3 country codes: CHN, China; IND, India; IDN, Indonesia; KOR, Republic of Korea; MYS, Malaysia; PHL, Philippine; THA, Thailand; VNM, Viet Nam. Source untraceable cases are indicated as domestic (Dom). Cases secondary or tertiary from a known confirmed case are indicated as related (R).
- d Negative for RT-PCR reaction; genotype unavailable.
| Age | No. | Male | No. of vaccinated/unknown/unvaccinated | IgM/IgG ratio <1 (%) | Imported no. (%) |
|---|---|---|---|---|---|
| ≤1 | 10 | 4 | 0/0/10 | 2 (20) | 10 (100) |
| 1–2 | 4 | 3 | 1/0/3 | 1 (25) | 3 (75) |
| 3–18 | 0 | 0 | – | – | – |
| 19–36a | 22 | 15 | 5/15/2 | 15 (68) | 9 (41) |
| 37–45 | 7 | 4 | 1/3/3 | 5 (71) | 7 (100) |
| Total | 43 | 26 | 7/18/18 | 23 (53) | 29 (67) |
- a Ages 19–36 are equivalent to a 1978–1995 birth cohort, estimated to have received two doses of MV-containing vaccine via the national immunization program and SIA.
Fourteen of the 43 cases (32.5 %) did not report traveling 7–21 days prior to the onset of rash and represent indigenous transmissions of MV (Table II), including four source-untraceable sporadic cases in 2012, two source-untraceable sporadic cases in 2013 (Table I, cases 6–9, 14–15), three secondary transmission cases with an epidemiological link to index cases from the Philippines (cases 27–28) and China (case 33), and one source-untraceable index case associated with four secondary cases from a family cluster in 2014 (Table I, cases 38–42). All 14 cases categorized as indigenous transmission were adults either reporting MMR vaccination or with an unknown history of vaccination, with the exception of one 18-month-old child who had received the first MMR dose approximately 3 months prior to infection.
Thirty-seven cases confirmed by RT-PCR in 2012–2014 were further characterized by MV genotyping. In 2012, five instances of genotype H1 and three of D8 were identified, while in 2013, five of H1 and two of D8 were found. In 2014, four genotypes—H1 (n = 5), D8 (n = 4), D9 (n = 1), and B3 (n = 12)—were detected (Table III). Various MV genotypes were detected in Taiwan between 2005 and 2014, including D4, B3, D5, D8, D9, G3, and H1 (Fig. 1). H1 was the most frequently detected genotype prior to 2010, whereas D9 was the most frequently detected MV genotype in 2010–2011. The H1 genotype rebounded in 2012 after an absence in 2011. In 2014, the B3 genotype was first detected in Taiwan and quickly became the most prevalent strain (Fig. 1 and Table III).
| Genotypes | |||||||
|---|---|---|---|---|---|---|---|
| Year | Country | H1 | D8 | D9 | B3 | NDa | Total |
| 2012 | China | 2 | 1 | 3 | |||
| India | 1 | 1 | |||||
| Thailand | 1 | 1 | |||||
| Domestic | 3 | 1 | 4 | ||||
| 2013 | China | 5 | 5 | ||||
| South Korea | 1 | 1 | |||||
| Domestic | 2 | 2 | |||||
| 2014 | China | 3 | 3 | 6 | |||
| China related | 1 | 1 | |||||
| Hong Kong | 1 | 1 | |||||
| Indonesia | 2 | 2 | |||||
| Malaysia | 1 | 1 | |||||
| Philippine | 5 | 1 | 6 | ||||
| Philippine related | 2 | 2 | |||||
| Vietnam | 1 | 1 | 2 | ||||
| Domestic | 1 | 1 | |||||
| Domestic related | 4 | 4 | |||||
| Total | 15 | 9 | 1 | 12 | 6 | 43 | |
- a ND: Genotype unavailable due to negative RT-PCRs.
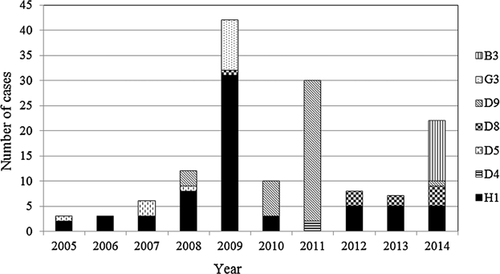
Based on the criterion of a travel history 7–21 days before the onset of rash for classification as an imported or domestic case [WHO, 2013], 11 cases of genotype H1 were imported from China (n = 10) and Vietnam (n = 1); six cases of genotype D8 were imported from Hong Kong (n = 1), India (n = 1), Thailand (n = 1), Indonesia (n = 2), and Vietnam (n = 1); one case of genotype D9 was imported from Malaysia; and seven cases of genotype B3 were imported from the Philippines (Table III). A country of import could not be identified for the other 11 cases, including three of genotype H1, three of genotype D8, and five of genotype B3, and these were classified as domestic cases (Table III).
To trace the origin of the 11 domestic cases, the 456 carboxyl terminal nucleotides encoding the nucleoprotein of the isolated MV strains were analyzed. Three viruses of genotype H1—MVs/Taipei.TWN/22.12, MVs/Taichung.TWN/23.12, and MVi/Taipei.TWN/24.12—with identical sequences were isolated from three cases (Table I; cases 6, 7, 8) with no epidemiological linkage. In 2013, MVs/Taoyuan.TWN/16.13 virus with an identical sequence was isolated in an imported case (Table I; case 14) from China (Fig. 2). When the sequences were BLAST searched against those in the National Center for Biotechnology Information (NCBI) database, we found that H1 virus had been detected in China and Japan between 2005 and 2008 (Fig. 2), suggesting that this virus has been maintained in circulation in China since 2005 (Fig. 2). Of the three cases of genotype D8 with untraceable sources, one virus (MVi/Taipei.TWN/36.12; case 9) was identical to an earlier strain (MVs/Taunton.GBR/27.12) from the United Kingdom. The other two identical viruses, MVs/Taipei.TWN/31.13/1 and MVi/Taipei.TWN/31.13/2, were isolated from two cases (cases 14 and 15) with no contact history. A BLAST search showed that this virus had appeared earlier in some European countries in 2012 and was reported in 2013 by additional countries in the Eastern Mediterranean Region (EMR) and the Western Pacific Region (WPR). This sequence was also identical to a virus, MVi/Pingtung.TWN/18.14 (case 29), imported from Vietnam in 2014, indicating that the D8 strain had spread globally (Fig. 2).
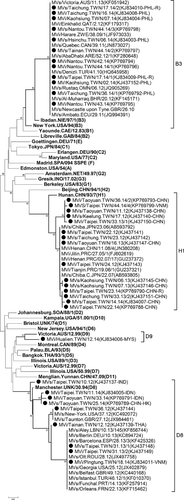
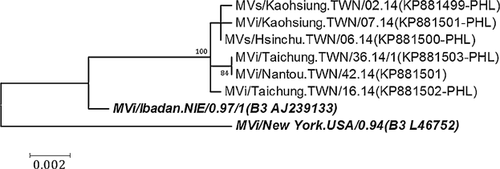
The 456 C-terminal nucleotides of five domestic cases of genotype B3 were identical to those of seven imported cases from the Philippines (Fig. 2). The 12 cases were from five independent importations and one family cluster without a traceable source. The sequences of H genes of viruses from five independent importations (Table I, cases 18–20, 26, and 36) and the index case of a family cluster (Table I, case 38) were sequenced and analyzed. The H gene sequences of MVi/Nantou.TWN/42.14 (index case) were identical to those of MVi/Taichung.TWN/36.14/1, but showed a 3–4 nucleotide divergence and a two amino acid difference from the other four B3 viruses from imported cases (MVs/Kaohsiung.TWN/02.14, MVs/Hsinchu.TWN/06.14, MVi/Kaohsiung.TWN/07.14, and MVi/Taichung.TWN/16.14; Fig. 3 and Supplementary Table II). The measles cases stemming from identical viruses occurred 6 weeks apart, implying that missing links in the transmission chain between the two cases cannot be excluded.
DISCUSSION
Measles is a childhood disease that primarily affects school and pre-school children prior to inoculation with the MMR vaccine. In Taiwan, over the 3 decades since the introduction of the national immunization program in 1978, new cases of measles are seldom reported in schoolchildren between the ages of 7 and 18, and the adult population—especially the cohort born between 1986 and 1992—have become the major victims in recent years [Cheng et al., 2013]. According to the national measles immunization plan, two supplementary rounds of immunization were undertaken: one implemented between 1992 and 1994 to target preschool through junior high school students (birth cohorts 1976/09–1990/09) and the other between 2001 and 2004 to target elementary school students (birth cohort 1990/09–1994/09) [Cheng et al., 2009]. The cohort born after 1978 should have received at least two doses of measles-containing vaccines but exact immunization records are, by and large, unavailable. In this study, serological data revealed that confirmed cases of measles among the 1978–1995 birth cohort (Table II; aged 19–36) more frequently exhibited an IgM/IgG index ratio <1 (68%; 15/22), representing a secondary immune response [Erdman et al., 1993; Rosen et al., 2014], compared to children younger than 1 year who had not yet received the MMR vaccination and were presumed to be non-immune and susceptible (20%, 2/10). In Taiwan, adult cases of measles presented distinct serological characteristics: negative, equivocal, or low positive levels of IgM antibodies, a high titer of IgG antibodies in serum, and PCR-positivity for MV in a throat swab or urine sample. Several cases in which a second serum sample was collected at least 7 days after the first showed IgM conversion from negative to positive and a significant rise in IgG, or a significant rise in IgG (data not shown) but negative IgM, lending support to the hypothesis of secondary measles vaccine failure [Edmonson et al., 1990; Ramsay and Brown, 2013].
A comparison of MV genotype data and the relevant countries over time indicated that dynamic changes in MV genotypes occurred in Taiwan and its neighboring countries. The major measles genotype in the Philippines was D3 before 2007, but shifted to D9 and G3 from 2007 to 2009 [Fuji et al., 2011; Rota et al., 2011], D9 from 2010 to 2012, and B3 from 2013 to 2014 [Takashima et al., 2015]. Meanwhile, the major genotypes in Indonesia were G3 and D9 before 2009 [Feng et al., 2006; Rota et al., 2011], and in 2014, two cases of genotype D8 (cases 22 and 29) featured a travel history to Indonesia (Table I). Genotype H1 was detected in many cases from Vietnam in 2009 [Cheng et al., 2011] but, by April 2014, one case from Vietnam exhibited the genotype D8. In Taiwan, the major genotype changed from H1 (mainly imported from China and Vietnam) prior to 2009 to D9 (imported from the Philippines) in 2010 [Cheng et al., 2013] and B3 (imported from the Philippines) in 2014. The genotype B3 first appeared in Taiwan and quickly became the most frequent strain in 2014. A similar virus appeared first on the African continent in 2009 and was reported in some European Countries in 2010, in the Americas in 2011, and in countries of the EMR in 2012 (Fig. 2). It was distributed further to countries in the WPR in 2013, and was associated with the measles outbreak in the Philippines in 2014 [Takahashi et al., 2014; Takashima et al., 2015; Yang et al., 2015]. This genotypic fluctuation could reflect the effort toward measles elimination. Large-scale supplementary immunization activity (SIA) has effectively terminated the endemic genotype in many countries, but if a high immunization coverage is not sustained in susceptible new birth cohorts or the older susceptible cohort, imported strains from neighboring countries can replace the strains of MV circulating previously.
Although humans are the only reservoir of MV, measles cases with an unidentified source of infection appear frequently. One possible explanation is that measles can be transmitted in the early stage of the disease prior to the onset of rash and through airborne spread without close contact, making it difficult to trace back to the previous link in the transmission chain. Another possibility is that an immunized person might be infected with MV and have milder symptoms resembling a cold, but nevertheless may possess the potential to transmit the virus. For example, consider the family cluster presented in Table I (cases 38–42): a child (case 42) was 18 months old and received the first dose MMR vaccine 3 months prior to infection. He had a fever and was notified after 1 week, due to a family cluster investigation in which he had contact with his aunt (a measles confirmed case) in the same household. His serum and throat swab were collected and tested for MV. His serum was negative for IgM and positive for IgG, but his throat swab was PCR-positive for MV and had an identical N gene sequence to the virus that infected his aunt, although no rash developed. When 20 possible contacts with the confirmed cases (case 42) were screened (defined as any person present in the same room or ward of the clinic 30 min before or 2 hr after an infectious measles patient), five children—part of an unvaccinated high-risk group under the age of 1—received intramuscular immunoglobulin (IMIG) for post-exposure prophylaxis. Although transmission of measles via an immunized carrier could not be verified, this finding provides a clue as to how untraceable measles cases could appear suddenly in a highly immunized community. If measles virus can survive temporarily in the respiratory tract of an immunized person, the odds of exposure of a naïve, susceptible host increase and may lead to virus transmission.
Collecting the appropriate samples, such as sera for the detection of IgM antibodies and throat swabs and urine samples for PCR tests, is important for confirming measles cases in a timely and accurate manner [Woo et al., 2010]. In this study, typical positive IgM in sera and positive PCR reactions in throat swabs or urine samples were found in 21 cases (48.8 %), a positive PCR reaction and negative or equivocal IgM were found in 16 cases (37.2 %), and positive IgM combined with negative PCR were found in six cases (14%). Of 43 confirmed MV cases, 16 and 6 cases would be misclassified if a throat swab and urine or sera, respectively, had not been collected. Measles is very contagious with an R0 range of 12–18 [Gay, 2004] and early identification of each measles case will aid in stopping its transmission and in facilitating effective intervention. The real-time collection of throat swabs and urine samples at the onset of rash helps to confirm cases with modified symptoms, especially young adults likely to have been partially immunized by one dose of MV-containing vaccine who may present with mild symptoms and might not show a typical IgM response in their sera. By extending the sequencing window of MV molecular epidemiology, clearly defined links provide insight into chains of transmission and help to document the circulation and importation of independent variants [Kessler et al., 2011].
The final steps toward eliminating measles will be difficult. SIA activity is an effective method to stop disease transmission and to quickly reduce its incidence. However, to reach the target goal of elimination, we must continuously invest money and manpower to maintain routine vaccination coverage and strengthen surveillance work. The budget for disease prevention and the maintenance of immunity might be reduced after outbreaks of measles cease. Because measles is highly contagious and has the potential to rebound, a high-level political commitment from every country is necessary for the success of regional and expanded programs leading to the global elimination of measles.
ACKNOWLEDGMENT
We thank the staff of local health bureaus for their assistance in the investigation and sample collection of suspected cases.




