Respiratory virus infections among children in South China
Abstract
Acute respiratory tract infection is an important cause of morbidity and mortality with a worldwide disease burden. This study aimed to determine the prevalence and clinical characteristics of children with viral-induced acute respiratory tract infection, in Southern China. Nasopharyngeal aspirate samples from 1,980 pediatric patients with suspected acute respiratory tract infection, and 82 samples from healthy subject controls were collected for routine examination at the Second Affiliated Hospital of Shantou University Medical College, from October 2007 to August 2011. Specimens were tested by multiplex polymerase chain reaction (mPCR). At least one or more viruses were detected from 1,087 samples (54.9%). These included laboratory confirmations for 446 respiratory syncytial virus (RSV), 386 influenza virus A (FluA), 315 human rhinovirus (HRV), 135 human bocavirus (HBoV), 119 Parainfluenza virus 3 (PIV3), 82 Parainfluenza virus 1 (PIV1), 66 adenovirus (ADV), 53 WU polyomavirus (WUPyV), 52 human metapneumovirus (hMPV), and 29 influenza virus B (FluB) samples. Samples from healthy subjects were negative for any virus. Of the patients with positive specimens, 107 (9.8%) were admitted to pediatric intensive care unit (PICU). Co-infection with at least two of the viral pathogens under study was observed in 325 of the 1,980 patients (16.4% of the total number of cases). These findings may help in the diagnosis of viral infections of the respiratory tract in children, and help to consider current and potential therapeutic approaches for the treatment of acute respiratory tract infection, and further respiratory complications. J. Med. Virol. 86:1249–1255, 2014. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Acute respiratory tract infection is an important cause of morbidity and mortality with a worldwide disease burden estimated at an exceedance of four million deaths per year [Seto et al., 2013]. Acute respiratory tract infection causes approximately 20% of deaths in pre-school children worldwide, with 90% of these deaths attributed to pneumonia. Common viral respiratory infections include respiratory syncytial virus (RSV), influenza types A and B (FluA and B), parainfluenza types 1 and 3 (PIV1 and 3), human rhinovirus (HRV), human metapneumovirus (hMPV), adenoviruses (ADV), WU polyomavirus (WUPyV), and human bocavirus (HBoV) [Bastien et al., 2007; Huiuijskens et al., 2012; Bierbaum et al., 2013; Kim et al., 2013]. These viruses cause acute respiratory tract disease in children, are often seasonal, and about 4–33% present with co-infection of at least one additional viral pathogen [Brunstein et al., 2008; Kaplan et al., 2008; Regamey et al., 2008; Sung et al., 2009]. Respiratory viruses can cause more serious clinical complications such as croup, bronchiolitis, and pneumonia, which often require hospitalization [García-García et al., 2012; Turner et al., 2013]. The aim of this study was to investigate the prevalence and clinical characteristics of children with viral acute respiratory tract infection in Southern China.
MATERIALS AND METHODS
Nasopharyngeal Aspirate Specimen Collection
From October 2007 to August 2011, nasopharyngeal aspirate specimens were collected from 1,980 pediatric patients (1,188 boys and 792 girls, age range 30 days to 12 years) with suspected acute respiratory tract infection. In addition 82 nasopharyngeal aspirate specimens were collected from healthy children for routine examination following outpatient service (49 boys and 33 girls, age range 3 months to 8 years). Written informed consent was obtained from the parents of each child, and the Ethical Committee of the Second Affiliated Hospital of Shantou University Medical College approved the experimental protocols.
The inclusion criterion was any acute respiratory tract infection, regardless of the reason for hospital admission. These samples were collected from a variety of geographical locations in China including Shantou, Chaozhou, Puning, Shanwei, and Jieyang. The nasopharyngeal aspirate samples were obtained within 24 hr of admission where possible, and immediately added to 3.0 ml of viral transport medium [Dulbecco's Modified Eagle Medium (DMEM) with 100 U/ml penicillin (Zhongnuo Pharmaceutical, Shijiazhuang, China); 100 µg/ml streptomycin (North China Pharmaceutical, Shijiazhuang, China); 2,000 U/ml amphotericin B (Sanland-chem International, Xiamen, China)] and viral transport medium was stored at −80°C until further processing. The final diagnosis was obtained from the discharge letter, and was made on the basis of both chest radiograph and clinical findings.
Viral Detection
Primers used for multiplex PCR amplification of respiratory viruses were selected based on the sequences available in GenBank. The primers were selected using Primer Express software (Primer 5.0 and Oligo 6.0) and synthesized by Invitrogen Life Technologies (Table I). All of the primer pairs were include in each PCR. The multiplex PCR used has been described previously [Templeton et al., 2004; Fan et al., 2007]. Nucleic acid preparation was performed using AxyPrep Body Fluid Viral DNA/RNA Miniprep Kit (Axygen, Shantou, Guangdong, China) according to the manufacturer's instructions. Briefly, individual NPA samples (200 µl) were mixed with an equal volume of viral lysis buffer and incubated for 5 min at room temperature. After adding 75 µl protein precipitation buffer, the mixture was centrifuged at 12,000g for 5 min. The supernatants were collected and mixed with 300 µl isopropanol + 1% acetic acid. The mixture was loaded on to a Miniprep column and centrifuged at 6,000g for 1 min, followed by sequential washings with 500 µl washing buffer and 800 µl of desalting buffer. Nucleic acids were eluted with 60 µl TE buffer, quantified, and stored at −80°C.
| Virus | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| FluA | AAGGGCTTTCACCGAAGAGG | CCCATTCTCATTACTGCTTC | 171 |
| FluB | GGGATATACGTAATGTGTTGT | GCACTGCCTGCTGTACACTT | 489 |
| PIV1 | CAGACGGCATATCTCCTCTGG | GGTATGAGAAATTACCGGGT | 307 |
| PIV3 | CTGGGCTTCATCAGTAGAGA | GATCTGTTGGTCACCACAAGA | 585 |
| RSV | TTTCCACAATATYTAAGTGTCAA | TCATCWCCATACTTTTCTGTTA | 155 |
| HRV | CGGTAATTTTGTACGCCAGTTT | GAAACACGGACACCCAAAGTAG | 501 |
| ADV | ATGTATTCCTTTTTCCGAAACTTCCA | GCCACATGGTGCGATCGCA | 248 |
| HBoV | GCAAACCCATCACTCTCAATGC | GCTCTCTCCTCCCAGTGACAT | 404 |
| hMPV | AACCGTGTACTAAGTGATGCACTC | CATTGTTTGACCG(A)GCC(A)CCA(G)TAA | 191 |
| WUPyV | TGTTACAAATAGCTGCAGGTC | GCATAATGGGGAGTACC | 217 |
Multiplex PCR involves reverse transcription of genomes from RNA viruses (FluA and B, PIV1 and 3, RSV, HRV, hMPV), followed by PCR amplification of the corresponding cDNA, and direct PCR amplification of genomes from DNA viruses (ADV, WUPyV, and HBoV). The respiratory viruses were confirmed by resolving PCR products on 1% agarose gels to confirm amplification of the predicted DNA. Sequencing was performed using a Beckman CEQ-8000 instrument and the identity of the PCR product sequences was confirmed using both BLAST and NCBI databases. Each PCR product was identified by size of the band following agarose gel electrophoresis and confirmed by DNA sequencing.
Statistical Analysis
Data were analyzed using statistical software (SPSS, version 13.0). For statistical analyses, the chi-squared test was applied. A P-value of less than 0.05 was considered significant.
RESULTS
Absolute and Relative Virus Frequency
From October 2007 to August 2011, nasopharyngeal aspirate specimens from 1,980 patients were collected; 150 of these patients admitted to pediatric intensive care units (PICU). Of the children diagnosed, 1,087 (54.9%) were positive for at least one virus, while none of the 82 healthy controls were positive. The positive specimens included the following viruses: RSV (446); FluA (386); HRV (315); hHBoV (135); PIV3 (119); PIV1 (82); ADV (66); WUPyV (53); hMPV (52); and FluB (29) (Fig. 1, Table II). Of the 1,087 patients with positive specimens, 107 (9.8%) were admitted to a pediatric intensive care unit with RSV (54) being the most prevalent (Tables II and III).
| Virus | Positive cases | Positive constituent ratio (%) | PICU cases | PICU constituent ratio |
|---|---|---|---|---|
| RSV | 446 | 41.03 | 54 | 12.11 |
| FluA | 386 | 35.51 | 8 | 2.07 |
| HRV | 315 | 28.98 | 50 | 15.87 |
| HBoV | 135 | 12.42 | 5 | 3.70 |
| PIV3 | 119 | 10.95 | 4 | 3.36 |
| PIV1 | 82 | 7.54 | 2 | 2.44 |
| ADV | 66 | 6.07 | 10 | 15.15 |
| WUPyV | 53 | 4.88 | 2 | 3.77 |
| hMPV | 52 | 4.78 | 2 | 3.85 |
| FluB | 29 | 2.67 | 3 | 10.34 |
| The general ward | PICU | χ2 | P-value | |
|---|---|---|---|---|
| No. of samples | 1,830 | 150 | ||
| Positive samples (%) | ||||
| Total | 980 | 107 | ||
| RSV | 392 (21.4%) | 54 (36%) | 16.88 | <0.01 |
| FluA | 378 (20.65%) | 8 (5.33%) | 20.74 | <0.01 |
| HRV | 265 (14.48%) | 50 (33%) | 36.83 | <0.01 |
| HBoV | 130 (7.10%) | 5 (3.33%) | 3.1 | >0.05 |
| PIV3 | 115 (6.28%) | 4 (2.67%) | 3.21 | >0.05 |
| PIV1 | 80 (4.37%) | 2 (1.33%) | 3.22 | >0.05 |
| ADV | 56 (3.06%) | 10 (6.67%) | 5.43 | <0.05 |
| WUPyV | 51 (2.79%) | 2 (1.33%) | 0.64 | >0.05 |
| hMPV | 50 (2.73%) | 2 (1.33%) | 0.58 | >0.05 |
| FluB | 26 (1.42%) | 3 (2%) | 0.046 | >0.05 |
- The boldness is to emphasize these results are meaningful.
Viral Co-Infections
Co-infection with at least two of the viral pathogens under study, was observed in 325 of the 1,980 patients (16.4% of the total number of cases); two pathogens were detected in 234 samples (11.8%), three pathogens in 68 samples (3.4%), four pathogens in 17 samples (0.9%), and six samples (0.3%) contained five viral pathogens. The most common pathogens in co-infected samples were PIV1 (68.5%), PIV3 (60%), FluB (58.6%), WUPyV (54.9%), FluA (52.1%), and HBoV (50.4%) (Table IV).
| Virus | Positive samples | Co-infected samples | Frequency (%) |
|---|---|---|---|
| RSV | 446 | 218 | 48.95 |
| FluA | 386 | 201 | 52.12 |
| HRV | 315 | 154 | 49.08 |
| HBoV | 135 | 68 | 50.37 |
| PIV3 | 119 | 71 | 60 |
| PIV1 | 82 | 57 | 68.51 |
| ADV | 31 | 66 | 48.21 |
| WUPyV | 53 | 29 | 54.90 |
| hMPV | 52 | 19 | 36.54 |
| FluB | 29 | 17 | 58.62 |
Correlation of Specific Viruses With Age Distribution
The age distribution of the patients from whom the viruses were detected was identical to the age distribution of the population sampled. Although RSV infection was detected in 26.5% of children, the frequency of RSV-positive samples decreased with increasing age. Among children in the first 6 months of life, RSV was the most common virus detected, infecting 30.5% (160/524) children (Table V). Only 19.6% of children older than 6 months of age were RSV positive (χ2 = 26.2, P < 0.05). While the percentage of FluA infected children rose from 18.6% to 22.9% after reaching the age of 3, the differences were not statistically significant. Similarly, no statistically significantly age group differences were observed in ADV, PIV1, PIV3, HBoV, hMPV, FluB, or WUPyV-infected individuals.
| Age | Samples | Positive samples (%) | Patients infected with each virus | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSV | HRV | FluA | ADV | PIV3 | PIV1 | HBoV | hMPV | FluB | WUPyV | ||||
| <6 months | 524 | 85.11 | 160 | 77 | 91 | 16 | 26 | 17 | 27 | 12 | 7 | 13 | 446 |
| 6 months–1 year | 466 | 82.83 | 103 | 75 | 80 | 17 | 31 | 21 | 32 | 10 | 6 | 11 | 386 |
| 1–3 years | 616 | 88.31 | 134 | 108 | 128 | 17 | 43 | 26 | 50 | 15 | 5 | 18 | 544 |
| 3–6 years | 262 | 84.35 | 34 | 37 | 64 | 8 | 16 | 12 | 17 | 15 | 8 | 10 | 221 |
| >6 years | 112 | 76.78 | 15 | 18 | 23 | 8 | 3 | 6 | 9 | 0 | 3 | 1 | 86 |
| Total | 1,980 | 85 | 446 | 315 | 386 | 66 | 119 | 82 | 135 | 52 | 29 | 53 | 1,683 |
Seasonal Distribution of Viruses
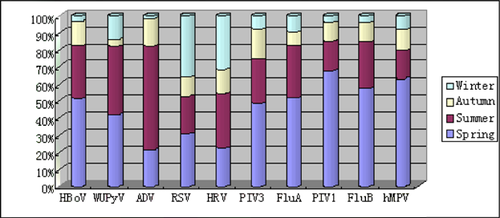
The virus detection rate was not distributed equally during different seasons (Fig. 2). RSV was detected mostly during the spring and winter, while ADV was primarily detected in the summer. HRV was detected in all seasons but detected mostly during the end of the winter and the beginning of spring. HBoV and WUPyV were detected primarily during the spring and summer, while PIV3, FluA, PIV1, FluB, and hMPV were primarily detected in the spring.
Clinical Characteristics of Patients With Viral Infection
The clinical symptoms and diagnosis of the patients with viral infection are shown in Tables VI and VII. Bronchopneumonia was the most common diagnosis for RSV, FluA, HRV, PIV3, PIV1, ADV, and WUPyV infection. HBoV and hMPV infection was more commonly associated with capillary bronchitis than bronchopneumonia. Fever, panting, and cough comprised the majority of clinical symptoms. Interestingly, two patients, one infected with WUPyV and one infected with HBoV, were diagnosed with severe encephalitis. In addition, two other patients, one infected with hMPV and one infected with HBoV, were diagnosed with respiratory failure.
| Virus | Number | BPN | Bronchiolitis | URTI | Capillary bronchitis | Laryngitis |
|---|---|---|---|---|---|---|
| RSV | 446 | 205 (45.9) | 40 (8.96) | 27 (6.05) | 130 (29.14) | 44 (9.86) |
| FluA | 386 | 154 (39.89) | 77 (30.05) | 38 (9.84) | 96 (24.87) | 21 (5.4) |
| HRV | 315 | 158 (50.16) | 35 (11.11) | 47 (14.92) | 40 (12.70) | 35 (11.11) |
| HBOV | 135 | 40 (29.62) | 23 (17.03) | 16 (11.85) | 48 (35.56) | 8 (5.92) |
| PIV3 | 119 | 61 (51.26) | 22 (18.49) | 24 (20.17) | 12 (10.08) | 0 |
| PIV1 | 82 | 40 (48.78) | 25 (30.48) | 15 (18.29) | 1 (1.22) | 1 (1.22) |
| ADV | 66 | 36 (54.54) | 13 (19.69) | 3 (4.54) | 12 (18.18) | 2 (3.03) |
| WUPyV | 53 | 25 (47.17) | 10 (18.87) | 5 (9.43) | 8 (15.1) | 5 (9.43) |
| hMPV | 52 | 18 (34.61) | 8 (15.38) | 5 (9.61) | 19 (36.54) | 2 (3.85) |
| FluB | 29 | 8 (27.59) | 10 (34.48) | 9 (31.03) | 1 (3.45) | 1 (3.45) |
| Total | 1,683 | 753 (44.74) | 273 (16.22) | 189 (11.22) | 349 (20.74) | 119 (7.07) |
- BPN, bronchopneumonia; URTI, upper respiratory tract infection.
| Virus | Number | Fever | Cough | Shortness of breath | Panting | Gastroenteritis | X-ray results | ||
|---|---|---|---|---|---|---|---|---|---|
| BPN changed | Bronchitis changed | No assay | |||||||
| RSV | 446 | 312 (69.95) | 446 (100) | 145 (31.51) | 215 (48.21) | 10 (2.24) | 205 (45.9) | 180 (40.36) | 61 (13.68) |
| FluA | 386 | 307 (79.53) | 380 (98.44) | 104 (26.94) | 113 (29.27) | 12 (3.11) | 154 (39.89) | 173 (44.82) | 59 (15.28) |
| HRV | 315 | 288 (91.43) | 267 (84.76) | 123 (39.05) | 196 (62.22) | 17 (5.40) | 149 (47.30) | 84 (26.98) | 82 (26.03) |
| HBoV | 135 | 114 (84.44) | 103 (76.30) | 96 (71.11) | 101 (74.81) | 9 (6.67) | 44 (32.59) | 63 (46.67) | 28 (20.74) |
| PIV3 | 119 | 97 (81.51) | 109 (91.60) | 46 (38.65) | 25 (21.01) | 8 (6.72) | 59 (49.58) | 36 (30.25) | 24 (20.17) |
| PIV1 | 82 | 77 (93.90) | 75 (91.46) | 5 (6.10) | 8 (9.76) | 10 (12.20) | 40 (48.78) | 26 (31.70) | 16 (19.51) |
| ADV | 66 | 66 (100) | 57 (86.36) | 17 (25.76) | 12 (18.18) | 4 (6.06) | 40 (60.60) | 25 (37.88) | 1 (1.51) |
| WUPyV | 53 | 30 (56.60) | 52 (98.11) | 27 (50.94) | 28 (52.83) | 5 (9.43) | 28 (52.83) | 18 (33.96) | 7 (13.21) |
| hMPV | 52 | 31 (59.61) | 40 (76.92) | 23 (44.23) | 25 (48.08) | 6 (11.54) | 19 (36.54) | 25 (48.08) | 8 (15.38) |
| FluB | 29 | 23 (79.31) | 28 (96.55) | 15 (51.72) | 9 (31.03) | 3 (10.34) | 10 (34.48) | 11 (37.93) | 8 (27.59) |
DISCUSSION
Acute respiratory tract infections are a persistent and significant public health problem. Worldwide, they cause a greater burden of disease than human immunodeficiency virus infection, malaria, cancer, or heart attacks. It has been postulated that they cause more illness and death than any other infection, and there has been little change in mortality due to acute respiratory tract infection for more than five decades [Mizgerd, 2006; Hasan et al., 2014]. Furthermore, respiratory infections are the most lethal of diseases in developing countries [Perdue et al., 2011].
This study describes the simultaneous detection of multiple respiratory pathogens with different clinical manifestations of acute respiratory tract infection in hospitalized children. An epidemiological investigation of ten viruses was conducted over 46 months. At least one viral pathogen was detected in 54.9% (1,087/1,980) of the nasopharyngeal aspirate specimens, a rate comparable to other studies (35–78%) [Bharaj et al., 2009; Sung et al., 2009; Bukhari and Elhazmi, 2013; Drieghe et al., 2014; Hasan et al., 2014]. This large number of specimens provided the study with an adequate database with which to make meaningful conclusions regarding the relative frequencies and seasonal distributions of the viruses detected and the statistical power to infer clinical correlations. Two or more pathogens were detected in 16.4% of the children, with some pathogens, such as HRV, WUPyV, and HBoV, being frequently identified as co-infections while others, including RSV and hMPV were more commonly detected as single infections. Co-infection was not associated with increased disease severity, an observation corroborated by other analyses [Canducci et al., 2008].
Patients with respiratory viral infection usually develop clinical symptoms, including fever, cough, panting, and shortness of breath. Such infections can lead to the development of bronchial pneumonia, bronchiolitis, and upper respiratory tract infection [Sung et al., 2009; Broor et al., 2013]. The study found fever and cough to be the most common clinical symptoms. Bronchopneumonia was the most common diagnosis for RSV, FluA, HRV, PIV3, PIV1, ADV, and WUPyV infection while capillary bronchitis was the most common diagnosis for HBoV and hMPV infection.
The most common pathogen detected in the study was RSV. RSV is the most common cause of childhood pneumonia worldwide [Zhang et al., 2010]. In the study, 36% of RSV-positive children were admitted to the pediatric intensive care unit, while 21.4% were admitted to the general ward setting. Immunity to RSV infection is not permanent with repeated infections throughout life being common. Furthermore, there is no effective vaccine and anti-viral treatment is not clinically indicated nor effective [Zhang et al., 2010]. RSV is particularly prevalent in infants below the age of 6 months [Reiche and Schweiger, 2009; Moore et al., 2013], an observation that our study corroborated.
Infections with FluA and HRV were also common and there were no age-specific differences between groups. For FluA infection, a small percentage of children required pediatric intensive care unit support. However, the incidence of influenza pediatric intensive care unit admissions decreased significantly compared with the general ward admissions. Conversely, the incidence of HRV in pediatric intensive care unit admissions increased significantly when compared with the general ward admissions.
Adenovirus infections are associated with different clinical syndromes. The majority of adenovirus infections result in tonsillopharyngitis and respiratory illness, particularly in children [Lu et al., 2013]. Furthermore, ADV are responsible for up to 10% of lower respiratory tract infections in children [Arden et al., 2006; Moura et al., 2007; Albarbi et al., 2012; Qurei et al., 2012]. In this study, a lower rate of ADV infection was found, as well as a difference between pediatric intensive care unit admissions and general ward admissions, even though ADV infections are being increasingly recognized as a cause of severe disease [Lynch et al., 2011].
This study identified increased detections with emerging viruses including HBoV, WUPyV, and hMPV. WU polyomavirus (WUPyV) was identified from lower respiratory tract samples in 2007 [Gaynor et al., 2007]. The prevalence rate of WUPyV infection varies from 0.4% to 13.9% among patients and asymptomatic individuals [Rao et al., 2011; Okada et al., 2013]. In this study, WUPyV infection rate was 2.7%. Previous studies have found that individuals with WUPyV infection are commonly co-infected with other viruses [Gaynor et al., 2007; Neske et al., 2008; Debiaggi et al., 2012]. In the current study, 29 of 53 patients with WUPyV infection were co-infected with at least one other virus.
Human bocavirus (HBoV) was first described in September 2005 [Allander et al., 2005]. The proportion of respiratory specimens from symptomatic hospitalized children that contain HBoV sequences has ranged from 1.5% to 19% [Allander et al., 2007; Deerojanawong et al., 2013; Levican et al., 2013]. In this study, the HBoV infection rate was 6.8%. According to the literature, HBoV DNA-positive acute respiratory tract infections occur in children across a range of months, with the peak season varying from year to year [Ahn et al., 2014]. Most researchers reporting from regions with temperate climates have observed a higher occurrence of HBoV detections during the winter and spring months [Smuts and Hardie, 2006; Tran et al., 2013]. This study found a relatively high occurrence of HBoV in the late spring and early summer in this study, consistent with previous findings [Choi et al., 2006]. This study detected one or more additional viruses in 50.4% (68/135) of HBoV-positive samples. Such high percentages have been reported by most studies that have investigated co-infections [Fry et al., 2007]. Previous studies have found that most children with HBoV, WUPyV, or hMPV infection had mild or moderate disease but in our studies, four patients who were positive for these emerging viruses, developed progressive diseases such as severe encephalitis or respiratory failure. It is important to examine further the propensity of HBoV, WUPyV, or hMPV to cause severe disease and the pathogenic mechanisms underlying this phenomenon.
CONCLUSIONS
Viral infections of the respiratory tract and the subsequent complications from these infections lead to a significant burden on healthcare systems throughout the world. Current treatments are less than ideal making clinical research and public health surveillance critical. These regionally specific findings will provide data to pediatricians and general practitioners to aid in the selection of appropriate therapies and to prevent the abuse of antibiotics. This study highlighted the incidence of respiratory pathogens in children in South China. These findings may help in the diagnosis of viral infections of the respiratory tract, in children. However, further studies with a large number of patients will increase our understanding of the pathogenesis of respiratory tract viral infections.




