Generation and epitope mapping of a sub-group cross-reactive anti-respiratory syncytial virus G glycoprotein monoclonal antibody which is protective in vivo
Abstract
Passively administered antibodies to conserved epitopes on the attachment (G) glycoprotein of human respiratory syncytial virus (hRSV) have potential in the immunoprophylaxis of human infections. This study set out to generate monoclonal antibodies (MAbs) recognizing all prevalent lineages of HRSV and capable of immunoprophylaxis in mice. Two murine MAbs of broad specificity for prevalent virus strains were generated by immunization of mice with hRSV of sub-group A followed by selection of hybridomas on recombinant G glycoprotein from a sub-group B virus. The anti-G hybridomas generated secreted antibody of high affinity but negligible neutralizing capacity one of which was tested in mice and found to be protective against live virus challenge. Western blotting and partial epitope mapping on transiently expressed G-glycoprotein fragments indicate that these antibodies recognize a complex epitope on the protein backbone of the molecule involving residues both C′- and N-terminal to the central conserved motif. J. Med. Virol. 86:1267–1277, 2014. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Human respiratory syncytial virus (hRSV) is a member of the Pneumovirus genus of the Paramyxoviridae. Two subgroups of virus (A and B) circulate within the community and each subgroup can be sub-divided into a number of antigenically distinct lineages with different lineages predominating in different epidemics [Sullender, 2000]. These viruses are responsible for a clinical spectrum in infancy, which includes severe lower respiratory tract disease. This is of particular concern in those who are premature or who have underlying diseases of the heart and lungs. Thereafter, re-infection is common throughout life, generally producing a predominantly upper respiratory tract infection although severe infection of the lung may occur in the immunosuppressed and the elderly [Hall, 2001].
In animal studies, protection against hRSV infection of the lower respiratory tract is effectively mediated by antibodies and passive administration of antibodies against both major surface glycoproteins of the virus, the fusion glycoprotein (F) and the large glycoprotein (G), have been shown to reduce virus titers in the lungs of rodents if given prior to infection [Taylor, 1994]. Antibodies can also protect against lower respiratory tract infection in humans and high serum antibody levels, acquired either transplacentally or by passive administration of intravenous immunoglobulin, reduce the incidence of infant infection requiring hospitalization [Glezen et al., 1981; Ogilvie et al., 1981; Holberg et al., 1991; Groothuis et al., 1993; Piedra et al., 2003; Stensballe et al., 2009]. Anti-F antibodies may play a central role in this protection as maternally acquired anti-F antibody levels have been correlated with milder disease [Piedra et al., 2003] and a humanized anti-F monoclonal antibody (MAb), Palivizumab, administered prophylactically has achieved a better than 50% reduction in hospital admissions for RSV infection of children at risk due to prematurity or underlying lung disease [Group, 1998].
The role of anti-G antibodies in human infection has been more difficult to define. Whilst the F glycoprotein is largely conserved, the G glycoprotein is highly variable and this variation, which is the principle source of the antigenic variation between virus lineages, has precluded assessment of the role of pre-existing anti-G antibodies on the outcome of natural infection with uncharacterized viruses. Antigenic analysis and structural studies of G have revealed a hairpin like structure embedded into the membrane via a C-terminal trans-membrane sequence [Langedijk et al., 1996]. The majority of MAbs generated against the protein are lineage- or strain-specific and map to the arms of the hairpin which comprise mucin-like polypeptides of highly variable sequence rich in potential sites for both O- and N-linked glycosylation the variable location of which may reveal or mask antibody epitopes [Martinez et al., 1997]. However, at the bend of the hairpin is a central region free from potential glycosylation sites. This comprises two short arms which differ between the two subgroups A and B but are conserved within them (Fig. 1) and to which sub-group specific MAbs map. The C-terminal arm carries overlapping heparin and fractalkine receptor CX3CR1binding motifs [Feldman et al., 1999; Tripp et al., 2001] suggesting that this region may be important in modulation of the innate immune responses to the virus. Separating the arms is a 13 amino acid motif which is conserved in all strains of the virus [Melero et al., 1997]. Although no function has been attributed to this motif, peptides with the same sequence are potent inhibitors of viral replication and NMR analysis of these peptides suggests that the motif adopts a disc-like structure with two hydrophobic faces suggesting involvement in receptor binding or oligomerization [Gorman et al., 2001].

 motif comprising amino acids conserved in all strains of hRSV.
motif comprising amino acids conserved in all strains of hRSV.  motifs comprising amino acids conserved among 100 sequences selected by a search of non-redundant protein sequences in the NCBI database using as the query sequence residues 151–221 of the G glycoprotein of the A2 strain for sub-group A and the corresponding region of the G glycoprotein of the 8/60 strain for sub-group B.
motifs comprising amino acids conserved among 100 sequences selected by a search of non-redundant protein sequences in the NCBI database using as the query sequence residues 151–221 of the G glycoprotein of the A2 strain for sub-group A and the corresponding region of the G glycoprotein of the 8/60 strain for sub-group B.  maximum and
maximum and  minimum length of the sequence required for binding of the specified MAb consistent with the data presented in Table III. Residues 172–187 are shown in bold.
minimum length of the sequence required for binding of the specified MAb consistent with the data presented in Table III. Residues 172–187 are shown in bold.Whilst, in animal models and in humans, sub-group specific responses to the epitopes which map to the sub-group specific flanks of this central region are easily demonstrated [Norrby et al., 1987; Murata et al., 2010; Murata and Catherman, 2012] the fully conserved 13 amino acid motif appears to be poorly antigenic. In animals polyclonal antibodies generated either to virus infection or to wild type or recombinant G glycoprotein have little sub-group cross reactivity [Stott et al., 1986; Johnson et al., 1987; Walsh et al., 1987; Murata and Catherman, 2012] and following primary infection of human infants, only lineage specific antibodies to the G glycoprotein were detected in the convalescent sera [McGill et al., 2004b]. However, Plotnicky-Gilquin et al. [1999] have demonstrated that antibody responses to a synthetic peptide corresponding to the central conserved motif do occur both in hyperimmunized mice and in older human convalescents and Palomo et al. [2000] have demonstrated antibodies which compete with monoclonal antibodies to this region in a number of human sera. Where present such responses may be beneficial as anti-G MAbs, generated in several laboratories and whose epitopes overlap this motif, have been shown to be protective in both cotton rats and mice reducing virus replication and, independently, pulmonary inflammation [Walsh et al., 1989; Plotnicky-Gilquin et al., 1999; Collarini et al., 2009; Haynes et al., 2009; Miao et al., 2009]. Understanding of the mechanism of action of these antibodies, however, remains incomplete and the protective epitopes involved have not been fully characterized.
This study set out to generate and characterize further cross-reactive MAbs recognizing the G glycoprotein of both hRSV sub-groups. Given the poor immunogenicity of the central conserved 13 amino acid motif, antibodies were generated by infection followed by hyper-immunization of mice with sub-group A virus. Sub-group cross-reactive hybridomas were generated from the immune spleens and selected on recombinant G glycoprotein from a B sub-group strain. The binding sites of two of these antibodies were partially mapped and the ability of the antibody with highest affinity to protect mice against intranasal challenge with live viruses of both sub-groups following passive administration was assessed.
MATERIALS AND METHODS
Cell Culture
HeLa and RK13 cell lines were provided by the Clinical Virology unit, The Royal Victoria infirmary, Newcastle upon Tyne and cultured in Eagle's minimal essential medium (EMEM; Cambrex Bio Science, Wokingham, Berkshire, UK) containing, 10% fetal calf serum (PAA Labs, Yeovil, Somerset, UK), 0.1 mg ml−1 penicillin, 0.1 mg ml−1 streptomycin, 0.02 mM L-glutamine and 0.02% NaHCO3 containing phenol red (Cambrex Bio Science). Cells were maintained in medium of the same composition but with only 2% fetal calf serum. Japanese quail fibrosarcoma cell line QT35 cells were a gift from Dr. B. Blacklaws (University of Cambridge, UK) and were cultured as described by Chiam et al. [2009].
Viruses and Antigens
Human respiratory syncytial virus strains A2 (lineage GA1) and 8/60 (lineage GB1) were as described by Taylor et al. [1992], kindly provided by E.J. Stott at unknown passage level and passaged up to six times in HeLa cells in this laboratory. HRSV Ncl/24702/96 (lineage GA2/3), Ncl/48/08 (lineage GA5), Ncl/25137/97 (lineage GA7), Ncl/21540/97 (lineage SAA1), Ncl/613/97 (lineage GB3), Ncl/17062/97 (lineage GB4), and Ncl/56/08 (lineage GBA4) were isolated from clinical material on HeLa cells in this laboratory and characterized by phylogenetic analysis as described previously [McGill et al., 2004a; Gaunt et al., 2011]. Virus infectivity was determined by the infectious focus assay as described by Gias et al. [2008]. Antigens for ELISA and for the immunization of mice were prepared from infected cell monolayers exhibiting extensive cytopathic effect. The cells were incubated in serum free medium for 24 hr prior to harvest after which they were scraped into the medium with a cell scraper and twice snap frozen in solid CO2/acetone and thawed. The lysate was sonicated while on ice, using a SANYO MSE Soniprep sonicator, with an amplitude of 10 microns, with 3 × 30 sec bursts with a 30-sec rest between each burst. Protein content was estimated using the BCA Novagen protein assay kit, (Merck Chemicals Ltd., Nottingham, UK).
Wild type vaccinia virus, strain Western reserve, and a vaccinia virus recombinant expressing the RSV subgroup B strain CH18537 G glycoprotein (vvGb) via the vaccinia virus early/late 7.5 kDa promoter [Wertz et al., 1987] were kindly provided by Dr Gail Wertz (The University of Virginia School of Medicine, Charlottesville, VA) and propagated in RK13 cells as described by Mackett [1995]. Modified vaccinia virus Ankara (MVA) was supplied by Dr. S. Bridge (Liverpool School of Tropical Medicine, Liverpool, UK). MVA-T7, a recombinant MVA expressing the bacteriophage λ T7 RNA polymerase under the control of the vaccinia virus P7.5 promoter [Sutter and Staib, 2003] was kindly supplied by Dr. G. Sutter (Institut für Infektionsmedizin und Zoonosen, Munich, Germany).
Peptide HFEVFNFVPCSIC, corresponding to residues 164–176 of the G glycoprotein of the A2 hRSV strain was synthesized by Eurogentech Ltd. (Seraign, Belgium). Peptide PCSICSNNPTCWAICK, corresponding to residues 172–187 of the same glycoprotein was synthesized by Dr J Gray (Institute for Molecular Biosciences, Newcastle University, UK).
Production of MAbs
Pilot experiments indicated that anti-RSV G glycoprotein antibody titers were low following hyperimmunization of mice with adjuvanted virus by the intramuscular route but could be increased by infection intranasally with live virus prior to immunization. Accordingly 6-week-old Balb/c mice were inoculated intranasally with 50 µl of an infected HeLa cell lysate containing 107 pfu of hRSV strain A2 under isofluorane anesthesia followed 14 and 28 days subsequently by sub-cutaneous inoculations with the same amount of the same antigen emulsified in incomplete Freund's adjuvant. Four months post-immunization each mouse received 20 µl of the same antigen without adjuvant intravenously. Four days later the immune mice were killed by cervical dislocation, and their spleens aseptically removed. Animals were handled under license from the UK Home Office and in compliance with UK law and local rules of Newcastle University.
Immune splenocytes were fused with P3-NS/1-Ag-1 mouse plasmacytoma cells (NS1) using polyethylene glycol and plated into HAT selective growth medium as previously described by Routledge et al. [1985]. Cultures were screened at 14 days for antibody by immunofluorescence staining of acetone fixed slides prepared, according to the methods described by Gardner and McQuillin [1980], from cultures of RK13 cells infected with recombinant vaccinia virus vvGb or, as a control, wild type vaccinia virus. Specific anti-G antibody positive colonies were repeatedly cloned by limit dilution until all growth positive wells were antibody positive.
Antibody Characterization
Antibodies were semi-purified from hybridoma supernatants prepared in the miniPERM system (Greiner Bio-one, Stonehouse, Gloucs, UK) by ammonium sulphate precipitation. Supernatants were mixed with an equal volume of saturated ammonium sulfate solution and incubated overnight at 4°C. Precipitated immunoglobulin was collected by centrifugation at 3,000g for 30 min, resuspended in approximately 1/50th of the original volume in PBS, dialyzed against three changes of PBS 4°C overnight and clarified by centrifugation at 3,000g for 30 min. Antibody isotypes were determined by IsoStrip mouse MAb isotyping Kits (Roche Products, Welwyn Garden City, UK) and immunoglobulin concentrations determined by isotype specific ELISA [West et al., 1994].
Infectious focus reduction neutralization assays were carried out on antibody preparations heated at 56°C for 30 min in the absence of complement as described by Marsh et al. [2007].
ELISA titrations of MAbs were carried out in 96 well ELISA plates (Nunc, Roskilde, Denmark) coated either with hRSV infected HeLa cell or control uninfected HeLa cell lysates at 20 µg/ml or with dilutions of synthetic peptide from 67 µg/ml in carbonate coating buffer pH9.6 according to the method described by Routledge et al. [1985]. Antibody affinities were determined on HRSV strain 8/60 infected cell lysate antigen as the reciprocal of the MAb concentration at half saturation as described by West et al. [1994]. Novel anti-G MAbs were compared with characterized anti-hRSV A2 G glycoprotein MAbs 4G4, 1C2 and 3F43 by the additive binding technique as described previously [Routledge et al., 1986; Mekseepralard et al., 2006]. The data was analyzed by the Student's paired t-test assuming uniform variance using Sigmaplot 11 (Systat Software, Hounslow, London, UK).
Western Blotting of Deglycosylated Viral Proteins
Confluent HeLa cell monolayers were inoculated with hRSV strain 8/60 at an MOI of five in maintenance medium. After 1 hr at 37o allowed for attachment the cultures were washed and re-incubated with maintenance medium containing 1 µg/ml of Tunicamycin (http://www.sigmaaldrich.com/catalog/product/sigma/t7765) and harvested 48 hr postinoculation. Mock infected and untreated virus infected cultures were prepared in parallel.
Harvested infected cells were boiled in sample buffer, resolved by SDS–PAGE under reducing conditions and Western blotted, as described by Nielsen et al. [2004] with either MAbs 133 or 21 and goat anti-mouse peroxidase conjugate (Novocastra laboratories, Newcastle upon Tyne, England) as primary and secondary antibody, respectively.
RSV G Gene Fragment Expression
The G gene of RSV strain Ncl/25137/97 (lineage GA7) was amplified by RT-PCR using primers designed from the known sequence of the gene (McGill et al., 2004b). These were gpGA_For, which introduces a Cfr91 restriction site before an ATG start codon eight residues upstream of the natural start codon silenced to TTG, and gpGA_Rev which introduced a Cfr91 site downstream of the stop codon (Table I). The amplicands were digested with Cfr91 and cloned into similarly digested plasmid pSC11 [Chakrabarti et al., 1985] to generate the plasmid 25173G2/pSC11_1. Full length and truncated forms of the G gene described in Table III were amplified from 25173G2/pSC11_1 using the appropriate combination of the forward and reverse primers described in Table I, where the forward primers incorporate the required gene initiation codon within an Nco1 site and the reverse primers incorporate a BamH1 site after the required termination codon. The amplicands were cloned into the vaccinia virus shuttle vector pTM3 (kindly provided by Dr. B. Moss, NIAID, Bethesda, USA) downstream of a T7 polymerase promoter and encephalomyocarditis virus internal ribosome entry site, with the initiation codon of the gene fragment coincident with that in the vector, which is embedded in the Nco1 site, to promote efficient translation [Elroy-Stein et al., 1989]. Recombinant plasmids were selected by colony PCR with primers pTMvec_F and pTMvec_R (Table I) which bind to sequences adjacent to the multiple cloning site in pTM3. For transient expression of the gene fragments, HeLa cell monolayers were infected with MVA-T at an moi of 3 and, after 30 min, transfected with 5 µg of recombinant pTM3 vectors carrying G fragments inserts or, as a control, a beta-galactosidase gene (β-gal) in 15 µg/ml Lipofectin reagent (Invitrogen Ltd., Paisley, UK). After incubation at 37°C for 24 hr the cells were suspended and slides prepared for immunofluorescence staining. Immunofluorescence staining was scored on a four point scale where “—” represents no discernable staining as seen on pTM3-βgal transfected MVA-T7 superinfected HeLa cells and “4+” represents staining equivalent to that of the Mab on pTM3-GA7 transfected/MVA-T7 superinfected cells (Fig. 2).
| Primer | Sequence (5′-3′) |
|---|---|
| gpGA_For | TAACCCCGGGGCAATTGCAAACATGTCCA |
| gpGA-Rev | TGGTCCCGGGTTTCCTCAGGAATACGCTTT |
| G-N1a_For | CGCAATTGCAACCATGGCCAAAACCAA |
| GN151a_For | AAGTCCACCACCATGGAACGCCAAAACAAACCACCAAAT |
| GN155a_For | AAACAACGCCCCATGGAACCACCAAATAAACCCAACAAT |
| GN158a_For | CAAAACAAACCCATGGATAAACCCAACAATGATTTTCAC |
| GC163_rev | GTTGAACACGGATCCTCAAAAATCATTGTTGGGTTTA |
| GC172b_Rev | GTTGCTGCAGGATCCCTAGGGTACAAAGTTGAACACTTC |
| GC177b_Rev | CCAGCAGGTGGATCCCTAGCATATGCTGCAGGGTACAAA |
| GC190b_Rev | CTTTCCAGGGGATCCTCATGGTATTCTTTTGCAGAT |
| G284b_Rev | TGTGATGGATCCTCAGATGTTGTATAGAC |
- Cfr91 (CCCGGG), Nco1 (CCATGG), and BamH1 (GGATCC) restriction sites are in italics. Initiation codons are boxed and stop codons underlined.
- a The residue encoded immediately downstream of the initiation codon and forming the N-terminus of the translated polypeptide.
- b The residue encoded immediately upstream of the stop codon and forming the C-terminus of the translated polypeptide.
Mouse Protection
Six- to eight-week-old female Balb/c mice were inoculated intravenously with 500 µg of ammonium sulfate purified antibody in a total volume of 100 µl in phosphate buffered saline and challenged intranasally 24 hr later with 106 pfu hRSV strain A2 or 8/60. Four days post-infection the lungs of each mouse were collected in 2 ml of maintenance medium (see Cell Culture above), macerated in Griffith's grinders and centrifuged for 5 min at 400g. Virus in the supernatant fluids was assayed as fluorescent foci (ffu) by the infectious focus assay described previously [Mekseepralard et al., 2006].
RESULTS
Generation and Characterization of Sub-Group Cross Reactive Anti-G Antibodies
Immune spleen cells from mice initially infected intranasally and then hyperimmunized sub-cutaneously with lysates of HeLa cells infected with sub-group A hRSV strain A2 were fused with NS1 myeloma cells and the resulting hybridomas were screened by immunofluorescence staining of RK13 cells infected with recombinant vaccinia virus expressing sub-group B hRSV strain CH18537 G glycoprotein (rvvGb). From 221 hybridomas generated, six produced anti-G monoclonal antibodies and from those hybridomas 21 and 133, both secreting IgG1, were cloned for further study.
The level of conservation of the MAb 21 and 133 epitopes was assessed by testing the reactivity of the MAbs with isolates of virus recovered from the population in Newcastle upon Tyne between 1996 and 2008 in immunofluorescence assays. HeLa cell cultures were infected with HRSV sub-group A viruses A2, Ncl/24702/96, Ncl/48/08, Ncl/17063/97, and Ncl/21540/97 belonging to lineages GA1, 2/3, 5, 7 and SAA1, respectively, and with sub-group B viruses 8/60, Ncl/613/97, Ncl/17062/97, and Ncl/56/08 belonging to lineages GB1, 3, 4, and BA-4. When exhibiting extensive cytopathic effect virus infected cells were harvested, dried onto glass slides, fixed in cold acetone and stained with hybridoma culture fluid by indirect immunofluorescence as described by Gardner and McQuillin [1980]. Both antibodies proved equally reactive with all sub-group A and sub-group B viruses (data not shown).
The affinity constants, 5.7 and 7.5 nanomoles respectively for MAbs 21 and 133, were determined by ELISA on antigen generated from hRSV strain 8/60 infected HeLa cells. These values are similar to that reported previously for the humanized anti-fusion glycoprotein antibody Palivizumab [Johnson et al., 1999]. The capacity of the MAbs to neutralize HRSV sub-group A strain A2 and sub-group B strain 8/60 was assessed by focus reduction neutralization test in HeLa cells in comparison with the neutralizing anti-F MAb Palivizumab. The neutralization capacity of both anti-G antibodies was negligible (Table II).
| MAb | Affinity constant (Ka) | Minimum neutralizing concentration (µg immunoglobulin/ml) | |
|---|---|---|---|
| A2 | 8/60 | ||
| 21a | 5.7 × 109 | 131 | >131 |
| 133a | 7.5 × 109 | 47 | 100 |
| PZ | 1.9 × 108 | 0.8 | 1.6 |
- The affinity constant is that reported by Wu et al. [2005] and was not determined in the present study. Neutralization titers were determined by the infectious focus reduction technique against the indicated virus strain in HeLa cells.
- PZ, palivizumab.
- a Affinity constants were determined on infected HRSV 8/60 infected cell lysate antigen as the reciprocal of the MAb concentration at half saturation as described by West et al. [1994].
The Effect of Glycosylation on Antibody Binding
A high proportion of the molecular mass of the G glycoprotein comprises oligosaccharide, predominantly O-linked, and binding of the majority of anti-G monoclonal antibodies is dependent upon the presence of this sugar [Palomo et al., 1991]. The conserved central region of the molecule however is free of potential glycosylation sites and antibodies recognizing epitopes within this region might be expected to bind independently of glycosylation. Collins and Mottet [1992] have demonstrated that a 36 kDa unglycosylated form of the G glycoprotein, aglycosyl Gm, can be generated by culture of the virus in the presence of the N-glycosylation inhibitor tunicamycin. Accordingly, in order to investigate whether the binding of the MAbs 21 and 133 is dependent upon glycosylation of the molecule, RSV strain 8/60 was grown in HeLa cells in the presence and absence of 1 µg/ml tunicamycin and the ability of the two MAbs to detect the 36 kDa aglycosyl form of the protein in infected cell lysates was determined by Western blotting. The results with both antibodies were similar (Fig. 3). Both antibodies stained a diffuse band of between 60 and 90 kDa in the untreated virus infected cell lysate corresponding to the fully glycosylated G and lower molecular weight bands similar to those previously described as partially glycosylated intermediates [Collins and Mottet, 1992]. In cultures prepared in the presence of tunicamycin the two major diffuse bands were reduced in intensity and both antibodies stained a sharp 36 kDa band corresponding to the electrophoretic mobility of the aglycosyl Gm protein [Wertz et al., 1985; Roberts et al., 1994]. A fainter 32 kDa band corresponding to the electrophoretic mobility of the soluble form of the G polypeptide lacking a membrane anchor, aglycosyl Gs [Roberts et al., 1994], was also stained. In addition, a 33 kDa band was detected which may correspond to the translational product from the second AUG start codon in the gene at Met48.
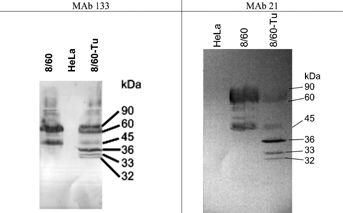
These results suggest that these MAbs are able to bind the peptide backbone of the G glycoprotein independent of carbohydrate.
Epitope Mapping by Additive Binding ELISA
Topographical mapping of the G epitopes of MAbs 133 and 21 can be achieved by additive binding ELISA [Routledge et al., 1986] in which an equal volume of homologous or heterologous MAbs are added to a solution of index MAb and allowed to bind to virus infected or control HeLa cell antigen in conditions of antibody excess. Immunoglobulin bound too the virus antigen is assessed by application of a secondary anti-mouse immunoglobulin peroxidase conjugate in an ELISA. Optical densities are corrected for binding to uninfected control cell antigen. In this assay, whilst addition of a heterologous MAb, recognizing an epitope independent of that of the index MAb, increases total immunoglobulin bound, addition of homologous MAb or one binding to an overlapping epitope results in no increase in binding. Antibodies with previously characterized sup-group A specific (1C2) and less broadly cross-reactive MAbs (4G4 and 3F43) known to bind distinct epitopes [Routledge et al., 1986; Morgan et al., 1987] were added individually to MAbs 21 or 131, and as controls to each other, and applied to replicate microtiter plate wells coated with hRSV A2 ELISA antigen where the concentration of each individual MAb and antigen had been previously chequerboard titrated to give an OD reading of approximately 1.0 in the presence of excess saturating antibody. Significant additive binding was observed between MAb 3F43 and all of the other MAbs and between 4G4 and 1C2 confirming that these three MAbs bind to distinct epitopes (Fig. 4a). There was no evidence of additive binding, however, between 133 and 21 or between either of these and 1C2 or 4G4 indicating that the epitopes of these MAbs are close together or overlapping (Fig. 4b).

Reactivity With Peptides
The cross-reactivity of MAbs21 and 133 MAbs for both sub-group A and sub-group B viruses suggests that the MAbs recognize the non-glycosylated 13 amino acid conserved region of the glycoprotein between residues 164 and 176. The ability of these antibodies to bind aglycosyl Gm and the failure of additive binding with MAb 1C2, which binds to the adjacent residues 174–187 [Trudel et al., 1991; Mekseepralard, 2002], is consistent with this hypothesis. To test this conclusion peptides corresponding to regions 164–176 and 172–187 were synthesized, bound to Maxisorb ELISA plates at equal concentrations and reacted with MAbs 21, 133, and 1C2, titrating down from approximately 20 µg immunoglobulin/ml. MAb 1C2, but neither MAb 133 or 21, bound strongly in a dose dependent manner to the sub-group specific region peptide, 172–187. None of the antibodies bound to the 164–176 conserved region peptide indicating that if the 21 and 133 epitopes involve this region it is insufficient alone to allow binding (data not shown).
Reactivity of MAbs With Truncated Forms of G Transiently Expressed From Recombinant Modified Vaccinia Virus Ankara (MVA)
Mabs 21, 133, 1C2, and 4G4, but not MAb 3F43, were found to react with the GA7 genotype HRSV strain A/Newcastle/25137/96 (data not shown). To further the definition of the epitopes of these MAbs the full GA7 G gene (codons 1–284) and forms of this G gene progressively truncated across the central conserved region from the 3′ terminus (message sense) corresponding to codons 1–190, 1–177, 1–173, and 1–163 or truncated from the 5′ end, corresponding to codons 151–284, 155–284, and 158–284, were generated by RT-PCR using the appropriate primers in Table I, cloned into the plasmid pTM3 downstream of a T7 polymerase promoter and transiently expressed in HeLa cells superinfected with MVA-T7 which expresses the T7 polymerase. Each MAb was tested for immunofluorescence staining of cells expressing these constructs, or control cells transfected with the empty plasmid pTM3 or infected with MVA-T7 without plasmid transfection (Table III). All of the constructs except one, corresponding to codons 1–163, were found to react strongly with at least one MAb, demonstrating expression of the truncated products.
| Truncation from N-terminus | Truncation from C-terminus | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mabs | 151–284 | 155–284 | 158–284 | 1–163 | 1–173 | 1–177 | 1–190 | 1–284 | pTM3 | MVA–T7 | pTM3–GA7 MVA–T7 | HeLa |
| 133 | 4+ | 4+ | — | — | — | — | 4+ | 4+ | — | — | 4+ | — |
| 21 | 4+ | 4+ | 1+ | — | — | — | 4+ | 4+ | — | — | 4+ | — |
| 1C2 | 4+ | 4+ | 4+ | — | — | — | 4+ | 4+ | — | — | 4+ | — |
| 4G4 | 4+ | 4+ | 1+ | — | 4+ | 4+ | 4+ | 4+ | — | — | 4+ | — |
- Immunofluorescence staining was scored on a four point scale where represents no discernable staining and 4+ represents staining equivalent to that of the Mab on pTM3-GA7 transfected/MVA-T7 superinfected HeLa cell (see Fig. 2).
MAb 4G4 bound specifically to all G polypeptides containing the codons 155–173, defining the maximum size of the 4G4 epitope (Fig. 1). Removal of the 5′ residues 155–157 in the 158–284 construct markedly reduced binding of the antibody. Removal of the 3′ residues to 163 also abrogated binding indicating that the 4G4 epitope overlaps the border between the 5′ sub-group specific and the fully conserved motif (164–177), however, as no antibodies binding to the 1–163 construct could be found, this conclusion requires confirmation. With this caveat, the minimum length of the 4G4 epitope consistent with these results would be 157–164.
MAb 1C2 bound to all polypeptides containing codons 158–190. Further loss of codons 178–190, from the 3′ end of this region abrogated binding which is consistent with the known binding of this MAb to the peptide corresponding to residues 172–184 and confirms that this epitope overlaps the border between the 3′ sub-group specific region and the 13 residue fully conserved region.
Both MAbs 21 and 133 bound to all peptides containing residues 155–190 indicating this as the maximum size of their epitope(s). Removal of either the 5′ residues 155–157, or the 3′ residues 178–190 adjacent to the fully conserved motif markedly reduced or abrogated binding. Thus, to be consistent with this data the minimum size of the linear sequence containing this epitope would be 157–178 (Fig. 1). This is consistent with the failure of these MAbs to bind a peptide corresponding only to the fully conserved 13 amino acids and suggests that the full epitope requires elements both C′- and N′-terminal to the conserved motif.
Mouse Protection
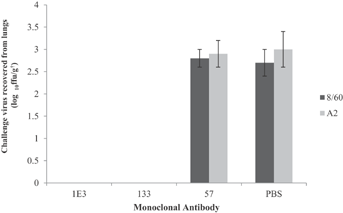
The antibody with the highest affinity, MAb 133, was selected for its ability to protect the lungs of mice against challenge with the two-subgroups of RSV, A and B. Groups of twelve mice were inoculated intravenously with 500 µg of one of the following - anti-G MAb 133, a positive control anti-fusion glycoprotein, MAb 1E3, previously shown to give complete passive protection of mice [Hayes et al., 1994], a negative control antibody MAb 57, an IgG1 MAb specific for the nucleoprotein of human metapneumovirus with no cross reactivity with hRSV [Fenwick et al., 2007], or with an equivalent volume of phosphate buffered saline. Six mice from each of the four treatment groups were challenged intranasally with 106 ffu of hRSV B strain 8/60 and six with a similar amount of hRSV A strain A2. Four days after challenge the lungs of the mice were collected, macerated and the macerates titrated for infectious virus. Similar titers of virus, approximately 3 log10 ffu/g, were found in the lungs of control mice treated with PBS or the irrelevant anti-HMPV antibody. However, no virus was recovered from mice receiving either anti-F MAb 1E3 or anti-G MAb 133 prior to infection with virus of either sub-group (Fig. 5). Thus, in this experiment MAb133 conferred protection against both virus subgroups comparable to that of the anti-F antibody.
DISCUSSION
After intranasal inoculation followed by repeated sub-cutaneous immunization of mice with live hRSV strain A2, hybridomas were selected on rvv-GB to yield one hybridoma secreting cross-reactive anti-G antibodies for every 37 screened. Two of these hybridomas were cloned for further assessment and found to be of affinity comparable to that of the anti-F antibody Palivizumab and to react with all of the lineages of hRSV of both sub-groups which have been circulating in Newcastle upon Tyne over recent years [McGill et al., 2004a; Gaunt et al., 2011].
Sub-group cross reactive anti-G MAbs have been recovered previously by a number of groups both as a minority species from virus immunized mice [Walsh et al., 1989; Martinez et al., 1997; Haynes et al., 2009] or from human peripheral blood leukocytes, where the frequency of B cells secreting cross-reactive anti-G antibodies was >10-fold lower than those recognizing any other RSV antigen [Collarini et al., 2009]. Additional antibodies have been generated by immunization of mice with synthetic peptides corresponding to non-glycosylated central conserved region of the protein [Plotnicky-Gilquin et al., 1999]. The epitopes of none of these antibodies have been fully characterized. The selection and sequencing of antibody escape mutants, where carried out, suggests that residues within the 13 residue, fully conserved motif (amino acids 164–176) are involved in antibody binding [Rueda et al., 1994; Martinez et al., 1997; Walsh et al., 1998] and some bind to a synthetic peptide corresponding to this motif [Plotnicky-Gilquin et al., 1999; Collarini et al., 2009]. However, neither of these approaches to epitope mapping is able to delineate the full extent of the target epitope which may extend beyond the conserved motif. Involvement of additional C′-terminal residues was indicated by the results of Rueda et al. [1994] who described a sub-group cross-reactive MAb which failed to bind to the conserved peptide. Sequencing of escape mutants generated to this MAb implicated residues both within the conserved motif but also the conserved cysteine residues 182 and 186 in the adjacent sub-group variable region.
The mapping of MAbs 133 and 21 here, confirm this conclusion but also implicate additional N′-terminal residues in binding of group cross-reactive antibodies. A preliminary assessment of the capacity of the cross-reactive antibodies to bind simultaneously to the G glycoprotein in additive binding assays, which give a preliminary assessment of the proximity of their epitopes, suggests that both bind close to the 1C2 epitope which has previously been shown to bind to a peptide, comprising amino acids 172–187, immediately C-terminal to the conserved 13 amino acid motif. Neither, however, proved capable of binding to peptide 164–176, the conserved motif, and reaction with truncated recombinant proteins indicated that the full epitope is carried on a larger fragment of the primary sequence than this. Thus, whist both antibodies bound to all truncated recombinant G fragments containing residues 155–190, deletion of an additional C-terminal 13 residues, 178–190, abrogated binding. Eight of these deleted residues are conserved between subgroups and these include the two conserved cysteines at 182 and 186, implicated in the binding of cross-reactive antibody by Rueda et al. [1994]. In the bovine RSV G glycoprotein these cysteines participate in a cystine-loop which comprises two anti-parallel alpha helices held together by two di-sulfide bridges with a reverse turn centered on a conserved asparagine residue and forming a surface structure with a diameter of approximately 20 Å. [Doreleijers et al., 1996]. This structure is similar in size to the epitope–paratope interface previously reported for a anti-lysozyme MAb [Barlow et al., 1986]. However, the full 21/133 epitope appears more complex than this as binding is abrogated by removal of amino acids 156–158, two of which, prolines 156 and 157, are conserved and which sit at the N-terminal edge of the central conserved region separating it from the highly glycosylated mucin-like stalk of the molecule [Langedijk et al., 1996]. These prolines have not hitherto been implicated in antibody binding and this data suggest that they are arranged close to the cystine-noose on the surface of the molecule a conclusion in accord with a model of the region generated by NMR [Gorman et al., 2001].
It remains unclear how an antibody interacting with residues at least 21 amino acids apart can retain specificity for a polypeptide linearized under reducing conditions in a Western blot.
That antibodies with this specificity could play a role in protection in vivo is indicated by the ability of MAb 133 to protect mouse lungs against live virus challenge. This property is shared with a number of other both sub-group cross-reactive and sub-group specific anti-G MAbs [Walsh et al., 1989, 1998; Mekseepralard et al., 2006; Haynes et al., 2009]. These anti-G MAbs are generally, but not-exclusively, non- or poorly neutralizing in cell culture. The mechanism of protection in vivo remains unclear but appears to be partially Fc-dependent and partially Fc-independent and may involve complement [Corbeil et al., 1996; Mekseepralard et al., 2006; Miao et al., 2009]. Whether antibodies of similar specificity can provide useful protection in human infants and what influence epitope specificity may have upon their efficacy remains to be determined.




