Predictive value of early viral dynamics during peginterferon and ribavirin combination therapy based on genetic polymorphisms near the IL28B gene in patients infected with HCV genotype 1b†
Conflict of interest: None.
Abstract
A study was carried out to determine whether early viral dynamics retain prediction of the outcome of peginterferon (PEG-IFN) and ribavirin combination therapy based on different genetic polymorphisms near the IL28B gene, the strongest baseline predictor of response to this therapy. A total of 272 patients infected with hepatitis C virus (HCV) genotype 1b were grouped according to genetic polymorphisms near the IL28B gene (rs8099917). The ability of reduced HCV RNA levels at 4 and 12 weeks after starting therapy to predict a sustained virologic response was evaluated based on these genotypes. Among patients with the TT genotype for rs8099917 (associated with a favorable response), the rates of sustained virologic response were higher in patients with a ≥3 log10 reduction in serum HCV RNA levels at 4 weeks after starting therapy (P < 0.0001). In contrast, among patients with the TG/GG genotype (associated with an unfavorable response), there were no differences in this rate based on the reduction in HCV RNA levels at 4 weeks. Early viral dynamics at 4 weeks after starting therapy retains its predictive value for sustained virologic response in patients with the TT genotype for rs8099917, but not in patients with the TG/GG genotype. Patients who are likely to achieve sustained virologic response despite unfavorable TG/GG genotype cannot be identified based on early viral dynamics during therapy. In contrast, lack of early virologic response at 12 weeks retains a strong predictive value for the failure of sustained virologic response regardless of IL28B polymorphisms, which remains useful as a factor to stop therapy. J. Med. Virol. 84:61–70, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
The current standard antiviral therapy for patients with chronic hepatitis C is combination therapy with peginterferon (PEG-IFN) and ribavirin [Ghany et al., 2009]. Although this treatment regimen has increased markedly the number of patients with a sustained virologic response, i.e., the eradication of hepatitis C virus (HCV), only 50% of patients infected with HCV genotype 1 achieved a sustained virologic response approximately.
Many investigators have examined factors that predict the treatment outcome of PEG-IFN and ribavirin combination therapy in patients infected with HCV genotype 1. In addition to the baseline factors, the response of HCV during combination therapy, i.e., the changes in serum HCV RNA levels after starting therapy, has been shown to be an important predictor of the treatment outcome [Zeuzem et al., 2001; Buti et al., 2002; Berg et al., 2003], with the emphasis on “response-guided therapy” [Lee and Ferenci, 2008; Marcellin and Rizzetto, 2008]. Recent reports have emphasized the importance of evaluating the viral dynamics at 4 weeks after starting therapy to predict a sustained virologic response. A rapid virologic response, in which serum HCV RNA is undetectable at 4 weeks after starting therapy, has been the strongest predictive factor of a sustained virologic response reportedly [Martinez-Bauer et al., 2006; Poordad et al., 2008; de Segadas-Soares et al., 2009; Martinot-Peignoux et al., 2009]. In addition, the predictive value of reduced serum HCV RNA levels at 4 weeks after starting therapy has been clarified further, and a ≥3 log10 reduction in HCV RNA levels at 4 weeks after starting therapy has high predictive value that a patient will achieve a sustained virologic response as a final outcome, even in the absence of a rapid virologic response [Toyoda et al., 2011].
In contrast, the lack of an early virologic response, defined as either undetectable serum HCV RNA or HCV RNA levels decreased by >2.0 log10 from the pretreatment level at 12 weeks after starting therapy, has been the most important predictor for the failure of a sustained virologic response in patients infected with HCV genotype 1 reportedly [Fried et al., 2002; Davis et al., 2003]. Therefore, treatment may be discontinued in patients without an early virologic response at 12 weeks of treatment, according to the recommendation in the AASLD guidelines [Ghany et al., 2009].
More recently, several studies reported that genetic polymorphisms near the IL28B gene (rs8099917, rs12979860) on chromosome 19 affect the virologic response to PEG-IFN and ribavirin combination therapy in patients infected with HCV genotype 1 [Ge et al., 2009; Suppiah et al., 2009; Tanaka et al., 2009; McCarthy et al., 2010; Rauch et al., 2010]. Furthermore, genetic polymorphisms near the IL28B gene are the strongest baseline predictive factor of the final outcome of combination therapy. An additional report showed the effects of genetic polymorphisms near the IL28B gene on HCV viral dynamics during PEG-IFN and ribavirin combination therapy [Thompson et al., 2010].
Although early HCV viral dynamics during therapy was shown originally to have a high predictive value for a sustained virologic response in HCV genotype 1-infected patients before genetic polymorphisms near the IL28B gene were linked to a therapeutic response, it is not clear whether early viral dynamics retain their predictive value in light of this additional information. The purpose of the present study was to investigate whether response-guided therapy based on viral dynamics at 4 or 12 weeks after initiating therapy retains its ability to predict the final outcome of PEG-IFN and ribavirin combination therapy after accounting for genetic polymorphisms near the IL28B gene.
MATERIALS AND METHODS
Patients and Treatment
Between January 2007 and June 2008, a total of 402 patients with chronic hepatitis C received antiviral combination therapy with PEG-IFN and ribavirin for HCV infection at the Ogaki Municipal Hospital or the Nagoya University Hospital. Among these patients, 272 were infected with HCV genotype 1b and had pretreatment HCV RNA levels >5.0 log10 IU/ml based on a quantitative real-time PCR-based method for HCV (HCV COBAS AmpliPrep/COBAS TaqMan System; Roche Molecular Systems, Pleasanton, CA; Lower limit of quantification, 1.7 log10 IU/ml: Lower limit of detection, 1.0 log10 IU/ml) [Colucci et al., 2007; Pittaluga et al., 2008]. This study did not include any patients infected with HCV genotype 1a because this genotype is not found in the general Japanese population.
All patients were given PEG-IFN alpha-2b (Pegintron, Schering-Plough, Tokyo, Japan) weekly and ribavirin (Rebetol, Schering-Plough, Kenilworth, NJ) daily. The PEG-IFN and ribavirin doses were adjusted based on the patient's body weight. Patients weighing ≤45 kg were given 60 µg of PEG-IFN alpha-2b once a week, those weighing >45 and ≤60 kg were given 80 µg, those weighing >60 and ≤75 kg were given 100 µg, those weighing >75 and ≤90 kg were given 120 µg, and those weighing >90 kg were given 150 µg. Patients weighing ≤60 kg were administered 600 mg of ribavirin per day, those weighing >60 and ≤80 kg were given 800 mg per day, and those weighing >80 kg were administered 1000 mg per day. The PEG-IFN and ribavirin doses were modified based on the manufacturer's recommendations. All patients were scheduled to undergo 48 weeks of treatment. The treatment duration was extended up to 72 weeks in some patients. In addition, treatment was discontinued before 48 weeks in some patients who had a low likelihood of achieving an eradication of HCV due to the presence of serum HCV RNA at 24 weeks after starting therapy.
A sustained virologic response was defined as undetectable serum HCV RNA at 24 weeks after ending the therapy. A patient was considered to have relapsed when serum HCV RNA was detectable between the end of treatment and 24 weeks after completing treatment, although serum HCV RNA was undetectable during and at the end of therapy. Patients were considered to have non-response if serum HCV RNA was detectable at 24 weeks after initiating therapy (i.e., null response or partial response according to the American guidelines [Ghany et al., 2009]). Patients were considered to have a rapid virologic response if they had undetectable serum HCV RNA at 4 weeks after starting therapy. An early virologic response was defined as the disappearance or decrease in serum HCV RNA levels by at least 2 log10 at 12 weeks after starting therapy. Patients were considered to have a complete early virologic response if serum HCV RNA was undetectable at 12 weeks after starting therapy and a partial early virologic response if the serum HCV RNA levels had decreased by at least 2 log10 at 12 weeks after initiating therapy. Patients were considered not to have an early virologic response if their HCV RNA levels did not decrease by more than 2 log10 at 12 weeks compared to the pretreatment levels. Patients were considered to have a slow virologic response if the serum HCV RNA became undetectable between 12 and 24 weeks.
The study protocol was in compliance with the Helsinki Declaration and was approved by the ethics committee of the Ogaki Municipal Hospital and the Nagoya University School of Medicine. Prior to initiating the study, each patient provided written informed consent to use the laboratory data, analyze genetic polymorphisms near the IL28B gene, and test stored serum samples.
Assessments of Serum HCV RNA Levels and Genetic Polymorphisms Near the IL28B Gene
After a patient provided informed consent, serum samples were obtained at the patient's regular hospital visits, just prior to initiating treatment, every 4 weeks during the treatment period, and during the 24-week follow-up period after treatment. Serum samples were stored at −80°C until further use. The HCV RNA levels were measured using a quantitative real-time PCR-based method for HCV (HCV COBAS AmpliPrep/COBAS TaqMan System).
Genotyping of rs 8099917 polymorphisms near the IL28B gene was performed using the TaqMan SNP assay (Applied Biosystems, Foster City, California) according to the manufacturer's guidelines. A pre-designed and functionally tested probe was used for rs8099917 (C__11710096_10, Applied Biosystems).
Statistical analyses
Quantitative values are reported as the mean ± SD. In between-group differences were analyzed by the chi-square test. Univariate and multivariate analyses using a logistic regression model were performed to identify factors that predict a sustained virologic response, including age, sex, body weight, serum alanine aminotransferase activity, serum aspartate aminotransferase activity, serum gamma-glutamyl transpeptidase levels, serum alkaline phosphatase values, serum albumin levels, total serum bilirubin values, white blood cell counts, hemoglobin, platelet counts, hepatitis activity grade (A0 and A1 vs. A2 and A3), liver fibrosis grade (F0 and F1 vs. F2 and F3), pretreatment HCV RNA levels (≥6.5 log10 vs. <6.5 log10), reduction in peginterferon dose and ribavirin dose, reduction in HCV RNA levels at 4 weeks after starting therapy (≥3 log10 vs. <3 log10), and the type of an early virologic response. All P-values are two-tailed, and P < 0.05 was considered significant statistically.
RESULTS
The characteristics of the patients examined in this study are shown in Table I. Liver histology was evaluated according to the METAVIR score [The French METAVIR Cooperative Study Group, 1994]. Although some patients had a reduction in their PEG-IFN and ribavirin doses during therapy, respectively, all patients except for those who discontinued the therapy had more than 80% adhesion to both the PEG-IFN and ribavirin regimens. No patients discontinued the therapy because of adverse effects. The treatment duration was extended up to 72 weeks in 51 of 71 patients (71.8%) who exhibited a slow virologic response. As a final outcome, 118 patients (43.4%) achieved a sustained virologic response, 84 patients (30.9%) relapsed, and the remaining 70 patients (25.7%) had no response.
| Age (years) | 56.0 ± 10.9 |
| Sex (female/male) | 139 (51.1)/133 (48.9) |
| Body weight (kg) | 57.8 ± 10.5 |
| Alanine aminotransferase (IU/L) | 64.6 ± 56.4 |
| Aspartate aminotransferase (IU/L) | 53.9 ± 42.7 |
| Gamma-glutamyl transpeptidase (IU) | 48.5 ± 43.9 |
| Alkaline phosphatase (IU/L) | 267.9 ± 101.3 |
| Albumin (g/dl) | 4.04 ± 0.37 |
| Total bilirubin (mg/dl) | 0.79 ± 0.30 |
| White blood cell count (/µl) | 4892 ± 1333 |
| Hemoglobin (g/dl) | 14.0 ± 1.3 |
| Platelet count (×103/µl) | 163 ± 51 |
| Liver histology-activity (A0/A1/A2/A3)* | 3 (1.2)/136 (55.3)/92 (37.4)/15 (6.1) |
| Liver histology-fibrosis (F0/F1/F2/F3)* | 27 (11.0)/114 (46.3)/70 (28.5)/35 (14.2) |
| Pretreatment HCV RNA concentration (log10 IU/ml) | 6.35 ± 0.79 |
| Reduction in the peginterferon dose | 81 (29.8) |
| Reduction in the ribavirin dose | 130 (47.8) |
| Final outcomes (sustained virologic response /relapse/ no response) | 118 (43.4)/84 (30.9)/70 (25.7) |
- HCV, hepatitis C virus.
- Percentages are shown in parentheses.
- * Liver biopsy was not performed in 26 patients.
Reduction in Serum HCV RNA Levels at 4 Weeks after Starting Therapy and Treatment Outcome According to Genetic Polymorphisms Near the IL28B Gene
An analysis of genetic polymorphisms at rs8099917 near the IL28B gene indicated that 207 patients (76.1%) had a TT genotype, 3 patients had a GG genotype (1.1%), and the remaining 62 patients were TG heterozygote (22.8%). Table II shows the comparison of the background characteristics between patients with the favorable TT genotype and those with the unfavorable TG/GG genotype. As reported previously [Abe et al., 2010], gamma-glutamyl transpeptidase level was higher significantly in patients with the TG/GG genotype. As a final outcome, the rate of a sustained virologic response was higher significantly in patients with the TT genotype. Among 207 patients with the TT genotype, serum HCV RNA became undetectable in 19 patients (9.2%) at 4 weeks after starting therapy (a rapid virologic response). In the remaining 188 patients, the decrease in serum HCV RNA levels at 4 weeks after starting therapy ranged from 0.12 log10 to 5.71 log10 (mean, 3.12 log10). The reduction in serum HCV RNA levels was ≥3 log10 in 98 patients (47.3%), <3 log10 and ≥2 log10 in 52 patients (25.1%), <2 log10 and ≥1 log10 in 23 patients (11.1%), and <1 log10 in 15 patients (7.3%). Figure 1A shows the rate of a sustained virologic response according to the reduction in HCV RNA levels at 4 weeks after starting therapy in patients with the TT genotype. The rates were higher significantly in patients who achieved a rapid virologic response or had a ≥3 log10 decrease in serum HCV RNA levels at 4 weeks compared to those with a <3 log10 decrease in serum HCV RNA levels (P < 0.0001). When a 3 log10 decrease in serum HCV RNA levels was defined as the cut-off point, 56.5% of patients were considered to have a ≥3 log10 decrease in serum HCV RNA levels. The sensitivity, specificity, positive predictive value, and negative predictive value for a sustained virologic response were 86.8, 75.2, 78.6, and 84.4%, respectively.
| Patients with TT genotype of rs8099917 (n = 207) | Patients with TG/GG genotype of rs8099917 (n = 65) | P-value | |
|---|---|---|---|
| Age (years) | 56.5 ± 10.4 | 54.4 ± 12.4 | 0.4112 |
| Sex (female/male) | 107 (51.7)/100 (48.3) | 32 (49.2)/33 (50.8) | 0.8384 |
| Body weight (kg) | 57.8 ± 10.9 | 57.8 ± 9.4 | 0.8361 |
| Alanine aminotransferase (IU/L) | 65.1 ± 53.3 | 62.8 ± 65.6 | 0.2548 |
| Aspartate aminotransferase (IU/L) | 53.6 ± 34.8 | 54.7 ± 62.0 | 0.3339 |
| Gamma-glutamyl transpeptidase (IU) | 44.2 ± 37.1 | 62.3 ± 59.0 | 0.0003 |
| Alkaline phosphatase (IU/L) | 263.1 ± 90.3 | 282.8 ± 129.9 | 0.3875 |
| Albumin (g/dl) | 4.04 ± 0.36 | 4.05 ± 0.43 | 0.8020 |
| Total bilirubin (mg/dl) | 0.79 ± 0.30 | 0.76 ± 0.32 | 0.3010 |
| White blood cell count (/µl) | 4826 ± 1333 | 5100 ± 1320 | 0.1608 |
| Hemoglobin (g/dl) | 13.9 ± 1.3 | 14.1 ± 1.4 | 0.3339 |
| Platelet count (×103/µl) | 161 ± 49 | 169 ± 57 | 0.3871 |
| Liver histology-activity (A0/A1/A2/A3)* | 2 (1.1)/98 (52.4)/74 (39.6)/13 (6.9) | 1 (1.7)/38 (64.4)/18 (30.5)/2 (3.4) | 0.3241 |
| Liver histology-fibrosis (F0/F1/F2/F3)* | 21 (11.2)/83 (44.4)/57 (30.5)/26 (13.9) | 6 (10.2)/31 (52.5)/13 (22.0)/9 (15.3) | 0.6401 |
| Pretreatment HCV RNA concentration (log10 IU/ml) | 6.37 ± 0.85 | 6.29 ± 0.55 | 0.0582 |
| Reduction in the peginterferon dose | 61 (29.5) | 20 (30.8) | 0.9644 |
| Reduction in the ribavirin dose | 101 (48.8) | 29 (44.6) | 0.5565 |
| Final outcomes (sustained virologic response /relapse/ no response) | 106 (51.2)/69 (33.3)/32 (15.5) | 12 (18.4)/15 (23.1)/38 (58.5) | <0.0001 |
- HCV, hepatitis C virus.
- Percentages are shown in parentheses.
- * Liver biopsy was not performed in 26 patients.
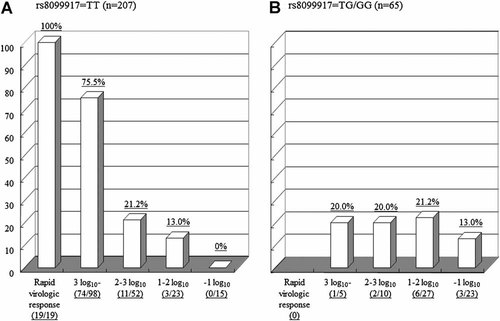
The rate of sustained virologic responses (%) based on the reduction in serum HCV RNA levels at 4 weeks after starting therapy. A: Patients with the TT genotype for rs8099917, (B) patients with the TG/GG genotype for rs8099917.
Among the 65 patients who had the TG/GG genotype, no patient achieved a rapid virologic response at 4 weeks after initiating therapy. The decrease in serum HCV RNA levels at 4 weeks after starting therapy ranged from 0.11 log10 to 4.75 log10 (mean, 1.66 log10). The reduction in serum HCV RNA levels at 4 weeks after starting the therapy were smaller in patients with the TG/GG genotype than those with the TT genotype (1.66 ± 1.02 log10 in patients with the TG/GG genotype vs. 3.12 ± 1.37 log10 in patients with TT genotype excluding RVR, P < 0.0001). The reduction in serum HCV RNA levels was ≥3 log10 in five patients (7.7%), <3 log10 and ≥2 log10 in 10 patients (15.4%), <2 log10 and ≥1 log10 in 27 patients (41.5%), and <1 log10 in 23 patients (35.4%). Figure 1B shows the rates of a sustained virologic response according to the reduction in HCV RNA levels at 4 weeks after starting therapy in patients with the TG/GG genotype. There were no differences in the rate of a sustained virologic response based on the reduction in HCV RNA levels at 4 weeks after starting therapy; the rate of a sustained virologic response remained at 20% approximately regardless of the reduction in HCV RNA levels in 42 patients with a ≥1 log10 reduction in serum HCV RNA levels.
Association Between an Early Virologic Response at 12 Weeks and Treatment Outcome Based on Genetic Polymorphisms Near the IL28B Gene
Figure 2 shows the rate of patients with the TT genotype or TG/GG genotype for rs8099917 who achieved a complete early virologic response, a partial early virologic response, and those who did not achieve early virologic response at 12 weeks after starting therapy based on the reduction in serum HCV RNA level at 4 weeks after initiating therapy. Nearly 75% of patients with the TT genotype whose HCV RNA levels were reduced by ≥3 log10 at 4 weeks after starting the therapy achieved a complete early virologic response. In contrast, 80% of patients with the TG/GG genotype whose HCV RNA levels were reduced by ≥3 log10 at 4 weeks after starting the therapy showed a partial early virologic response. The majority of patients with the TT or TG/GG genotypes achieved a partial early virologic response when their reduction in HCV RNA levels was <3 log10 and ≥2 log10 or <2 log10 and ≥1 log10.
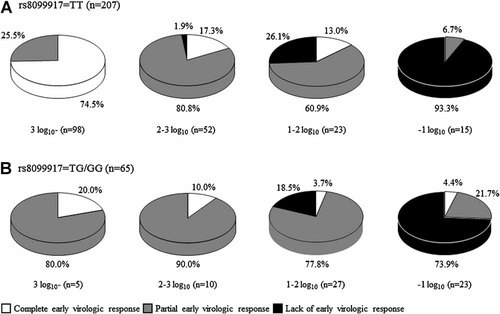
The association between the virologic responses at 12 weeks after starting therapy and the reduction in serum HCV RNA levels at 4 weeks after starting therapy. A: Patients with the TT genotype for rs8099917, (B) patients with the TG/GG genotype for rs8099917.
Figure 3 shows the rates of a sustained virologic response according to the type of early virologic response in patients with the TT genotype (Fig. 3A) and TG/GG genotype (Fig. 3B). Among patients with the TT genotype, the rate of sustained virologic response was significantly higher in patients with a complete early virologic response than in those with a partial early virologic response (P < 0.0001). In contrast, there was no difference in the rate of a sustained virologic response between patients with a complete early virologic response and those with a partial early virologic response (P = 0.8917) among patients with the TG/GG genotype. None of the patients with the TT genotype or TG/GG genotype who yielded a lack of an early virologic response reached a sustained virologic response.
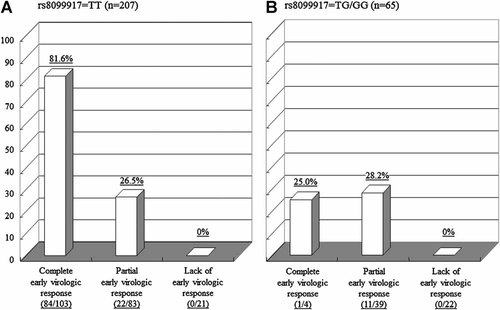
The rate of sustained virologic responses based on the type of early virologic response. A: Patients with the TT genotype for rs8099917, (B) patients with the TG/GG genotype for rs8099917.
Univariate and Multivariate Analyses for Factors Associated With a Sustained Virologic Response to Peginterferon and Ribavirin Combination Therapy in Patients With the TT and the TG/GG Genotype for the rs8099917
Univariate and multivariate analyses were conducted for factors associated with a sustained virologic response based on different genetic polymorphisms near the IL28B gene. In patients with the TT genotype, the factors that were associated with a sustained virologic response included serum alkaline phosphatase levels, serum albumin, platelet counts, hepatitis activity grade, liver fibrosis grade, reduction in HCV RNA levels at 4 weeks after starting therapy, and a complete early virologic response based on a univariate analysis (Table IIIA). In a multivariate analysis, the serum albumin levels, reduction in HCV RNA levels 4 weeks after starting therapy, and a complete early virologic response were independent factors that were significantly associated with a sustained virologic response (Table IIIB). A reduction in HCV RNA levels 4 weeks after starting therapy was the strongest factor that affected a sustained virologic response. In patients with the TG/GG genotype, the factors that were associated with a sustained virologic response included patient age, platelet counts, and pretreatment HCV RNA levels based on a univariate analysis (Table IIIA). A reduction in the HCV RNA levels at 4 weeks after starting therapy was not associated with a sustained virologic response. In a multivariate analysis, patient age and pretreatment HCV RNA levels were independent factors that were significantly associated with a sustained virologic response (Table IIIC).
| (A) Univariate analyses | P-value | |
|---|---|---|
| Patients with TT genotype of rs8099917 (n = 207) | Patients with TG/GG genotype of rs8099917 (n = 65) | |
| Age (years) | 0.0505 | 0.0007 |
| Sex (female/male) | 0.1830 | 0.2296 |
| Body weight (kg) | 0.6891 | 0.2456 |
| Alanine aminotransferase (IU/L) | 0.7988 | 0.4032 |
| Aspartate aminotransferase (IU/L) | 0.5021 | 0.1705 |
| Gamma-glutamyl transpeptidase (IU) | 0.6340 | 0.6648 |
| Alkaline phosphatase (IU/L) | 0.0315 | 0.0599 |
| Albumin (g/dl) | 0.0002 | 0.6594 |
| Total bilirubin (mg/dl) | 0.2929 | 0.7130 |
| White blood cell count (/µl) | 0.2508 | 0.5549 |
| Hemoglobin (g/dl) | 0.0847 | 0.2289 |
| Platelet count (×103/µl) | 0.0454 | 0.0411 |
| Liver histology-activity (A0–1/A2–3) | 0.0445 | 0.1117 |
| Liver histology-fibrosis (F0–1/F2–3) | 0.0002 | 0.2283 |
| Pretreatment HCV RNA concentration (≥6.5 log10 vs. <6.5 log10) | 0.5279 | 0.0379 |
| Reduction in the peginterferon dose | 0.4316 | 0.5563 |
| Reduction in the ribavirin dose | 0.1823 | 0.4272 |
| Reduction in HCV RNA levels at 4 weeks after starting the therapy (≥3 log10 vs. < 3 log10) | <0.0001 | 0.9265 |
| Early virologic response (complete vs. partial) | <0.0001 | 0.9777 |
| Early virologic response (partial vs. non) | 0.8632 | 0.0686 |
| (B) Multivariate analyses: Patients with TT genotype of rs8099917 | P-value | Odds ratio (95% confidence interval) |
|---|---|---|
| Alkaline phosphatase (IU/L) | 0.2617 | |
| Albumin (g/dl) | 0.0365 | 28.287 (1.4107–755.41) |
| Platelet count (×103/µl) | 0.2599 | |
| Liver histology-activity (A0–1/A2–3) | 0.6678 | |
| Liver histology-fibrosis (F0–1/F2–3) | 0.2307 | |
| Reduction in HCV RNA levels at 4 weeks after starting the therapy (≥3 log10 vs. <3 log10) | <0.0001 | 16.029 (6.8593–40.406) |
| Early virologic response (complete vs. partial) | 0.0224 | 0.3685 (0.1557–0.8749) |
| (C) Multivariate analyses: Patients with TG/GG genotype of rs8099917 | P-value | Odds ratio (95% confidence interval) |
|---|---|---|
| Age (years) | 0.0022 | 0.0034 (0.0000–0.0840) |
| Platelet count (×103/µl) | 0.3344 | |
| Pretreatment HCV RNA concentration (≥6.5 log10 vs. <6.5 log10) | 0.0304 | 0.0548 (0.0020–0.4950) |
- HCV, hepatitis C virus.
Characteristics of Patients who Achieved a Sustained Virologic Response to the Combination Therapy Despite the Unfavorable TG/GG Genotype Near the IL28B Gene
Table IV shows the characteristics of 12 patients who achieved a sustained virologic response despite having the unfavorable TG/GG genotype for rs8099917 near the IL28B gene. All but one patient was under 60 years old and had liver fibrosis not more than grade 2 (one patient did not undergo a liver biopsy). Except for one patient, the reduction in the serum HCV RNA levels at 4 weeks after starting therapy was less than 3 log10 and all but one patient showed a partial early virologic response at 12 weeks after starting the therapy. In all 11 patients with a partial early virologic response, the serum HCV RNA was undetectable up to 24 weeks after starting the therapy. All but one patient extended the treatment duration from 48 to 72 weeks (two patients discontinued therapy at 60 weeks during the extended treatment period). When the characteristics of patients who achieved a sustained virologic response were compared between those with the unfavorable TG/GG genotype and those with the favorable TT genotype, patients with the TG/GG genotype were younger (41.8 ± 14.4 years vs. 55.1 ± 10.4 years, P = 0.0023) and had lower pretreatment HCV RNA levels (5.91 ± 0.44 log10 IU/ml vs. 6.21 ± 1.05 log10 IU/ml, P = 0.0199).
| Age (years) | Sex | Liver histology | Pretreatment HCV RNA level (log10 IU/ml) | HCV RNA reduction at 4 weeks | Response at 12 weeks | HCV RNA became undetectable (weeks) | Treatment duration (weeks) | |
|---|---|---|---|---|---|---|---|---|
| 1. | 31 | Female | A1/F1 | 6.13 | 2.19 | partial EVR | 20 | 48 |
| 2. | 55 | Male | A1/F1 | 5.80 | 1.77 | partial EVR | 16 | 72 |
| 3. | 57 | Female | A1/F1 | 5.58 | 3.01 | partial EVR | 16 | 72 |
| 4. | 57 | Female | A1/F1 | 6.21 | 1.81 | partial EVR | 20 | 72 |
| 5. | 62 | Male | N.D. | 6.23 | 1.13 | partial EVR | 24 | 72 |
| 6. | 21 | Male | A1/F2 | 6.04 | 1.83 | partial EVR | 24 | 72 |
| 7. | 42 | Male | A1/F1 | 6.27 | 0.57 | partial EVR | 24 | 72 |
| 8. | 29 | Female | A1/F2 | 5.83 | 1.83 | partial EVR | 20 | 60 |
| 9. | 52 | Male | A1/F0 | 5.91 | 2.12 | complete EVR | 12 | 48 |
| 10. | 40 | Male | A2/F1 | 5.84 | 1.34 | partial EVR | 20 | 72 |
| 11. | 27 | Male | N.D. | 5.63 | 0.42 | partial EVR | 24 | 72 |
| 12. | 28 | Male | A1/F0 | 6.59 | 0.76 | partial EVR | 20 | 60 |
- N.D., not done; HCV, hepatitis C virus; EVR, early virologic response.
DISCUSSION
Several previous studies reported that patients who achieved a rapid virologic response, in which serum HCV RNA become undetectable at 4 weeks after starting therapy, had a high likelihood of achieving a sustained virologic response [Martinez-Bauer et al., 2006; Poordad et al., 2008; de Segadas-Soares et al., 2009; Martinot-Peignoux et al., 2009]. In addition, several recent studies reported the predictive value of the degree of reduction in serum HCV RNA levels at 4 weeks after starting therapy [Yu et al., 2007; Huang et al., 2010; Toyoda et al., 2011]. Therefore, the viral dynamics of HCV at 4 as well as 12 weeks after starting therapy is important for response-guided therapy.
Genetic polymorphisms near the IL28B gene have emerged as the strongest predictive factor of a sustained virologic response in patients infected with HCV genotype 1 [Hayes et al., 2011; Kurosaki et al., 2011]. In addition, Thompson et al. [2010 reported that genetic polymorphisms near the IL28B gene were associated strongly with early viral dynamics during PEG-IFN and ribavirin combination therapy. These findings raised an important issue of whether response-guided therapy, based on the reduction in serum HCV RNA levels at 4 or 12 weeks after starting therapy, retains a predictive value when considering genetic polymorphisms near the IL28B gene.
In the present study, the predictive value of the decrease in serum HCV RNA levels was evaluated at 4 and 12 weeks after starting therapy in Japanese patients infected with HCV genotype 1b based on genetic polymorphisms near the IL28B gene. Consistent with previous reports, patients with the TG/GG genotype for rs8099917 had a smaller reduction in serum HCV RNA levels at 4 weeks after starting treatment (P < 0.0001), which indicates an unfavorable response to the combination therapy. Patients with the TT genotype for rs8099917, which is associated with a favorable response to the combination therapy, exhibited a significant difference in the rate of a sustained virologic response based on the reduction in serum HCV RNA levels at 4 weeks after initiating the therapy. Patients with a rapid virologic response or with a ≥3 log10 reduction in HCV RNA levels had a higher likelihood of achieving a sustained virologic response.
In contrast, these factors did not have any predictive value in patients with the TG/GG genotype. Only 18.5% of patients achieved a sustained virologic response (12 of 65 patients), and it was difficult to identify these patients based on the reduction in HCV RNA levels at 4 weeks or the type of an early virologic response at 12 weeks after starting therapy. Patients who achieved a sustained virologic response, despite the TG/GG genotype for rs8099917, were identified among those with a <2 log10 and ≥1 log10 or even <1 log10 reduction in HCV RNA levels at 4 weeks after starting therapy. Interestingly and paradoxically, the possibility of a sustained virologic response can be expected in patients with a <1 log10 reduction in HCV RNA levels at 4 weeks after starting therapy only when they have the unfavorable TG/GG genotype.
In the evaluation at 12 weeks after starting therapy, patients with the TT genotype who achieved a complete early virologic response had a higher rate of a sustained virologic response significantly than patients who achieved a partial early virologic response, whereas this difference was not found in patients with the TG/GG genotype. No patients who failed to achieve an early virologic response achieved a sustained virologic response regardless of the genetic polymorphisms near the IL28B gene. Thus, the lack of an early virologic response retained a strong predictive value for the failure of achieving a sustained virologic response. This result supports the recommendation in the AASLD guidelines, in which treatment may be discontinued in patients without an early virologic response at 12 weeks of treatment.
The characteristics of patients who achieved a sustained virologic response despite the unfavorable TG/GG genotype were younger in age and lower pretreatment HCV RNA levels. Most patients with the TG/GG genotype who achieved a sustained virologic response showed a partial early virologic response and extended the treatment duration. It was difficult to identify these patients according to viral dynamics at 4 or 12 weeks after starting therapy.
There are several limitations in this study. Some patients with a slow virologic response did not have their treatment period extended from 48 to 72 weeks. This is because the effectiveness of a 72-week combination therapy regimen in patients with HCV genotype 1 with a slow virologic response [Berg et al., 2006; Pearlman et al., 2007] had not been established in Japan in the earlier part of this study. This fact might have influenced the treatment outcome especially in patients with the unfavorable TG/GG genotype. Another limitation is a smaller sample size of patients with the TG/GG genotype in comparison to that of patients with the TT genotype. This sample size could have caused the lack of statistical significance in the rate of a sustained virologic response according to the reduction in HCV RNA levels at 4 weeks after starting therapy or according to the type of an early virologic response in patients with the TG/GG genotype. In addition, the data were based on Japanese patients infected with HCV genotype 1b. Therefore, these results should be confirmed in other ethnicities and patients infected with HCV genotype 1a.
In conclusion, among patients infected with HCV genotype 1b with the TT genotype for rs8099917, a rapid virologic response or a ≥3 log10 reduction in HCV RNA levels at 4 weeks after starting therapy, or a complete early virologic response indicate strongly that these patients will achieve a sustained virologic response as a final outcome for PEG-IFN and ribavirin combination therapy. Early viral dynamics retain the predictive value in this patient subpopulation. A reduction in HCV RNA levels at 4 weeks after starting therapy or the type of an early virologic response does not predict the likelihood that patients with the TG/GG genotype will achieve a sustained virologic response. In contrast, the lack of an early virologic response retains a strong predictive value for the failure to achieve a sustained virologic response regardless of IL28B polymorphisms, which remains useful as a factor to stop therapy.




