Longitudinal analysis of frequency and reactivity of epstein–barr virus-specific T lymphocytes and their association with intermittent viral reactivation
Abstract
Persistent Epstein–Barr virus (EBV) infection is controlled tightly by virus-specific T cells. EBV infection is reactivated intermittently over time, even in apparently healthy carriers. Changes in frequency and reactivity of memory T cells, particularly of CD8+ origin, have not been assessed in this context. It is hypothesized that viral reactivation is facilitated by diminished EBV-specific T-cell immunity. To this end, blood samples from 14 healthy donors were collected at irregular time intervals for a period of about 1 year. Samples were screened for both EBV plasma viremia and increases in viral load in PBMCs as parameters of EBV reactivation. PBMCs were subject to IFN-γ ELISPOT analysis using the autologous EBV-transformed lymphoblastoid cell line (EBV-LCL) or appropriate HLA class I-restricted EBV peptides as stimulators. Frequencies of epitope-specific CD8+ T cells were monitored further using HLA tetramers and flow cytometry. Twelve of 14 donors exhibited signs of asymptomatic EBV reactivation. Viral reactivation was accompanied by either substantially decreased IFN-γ responses against autologous EBV-LCL (eight of 12 study participants) and/or increased responses against particular EBV peptides (six of 12 donors). In seven persons with HLA-A2 and/or -B8 alleles numbers of HLA tetramer-positive CD8+ T cells also varied over time, but showed no correlation to episodes of detectable viral activity. In summary, IFN-γ reactivity of EBV-specific T cells is not constant. Viral reactivation is detected preferably at times of diminished EBV-LCL-specific cellular immunity. However, increased reactivity of single immunodominant CD8+ EBV-specific T-cell clones may occur in response to virus replication. J. Med. Virol. 84:119–131, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
EBV belongs to the family of human Herpesviruses and as such is able to persist in its host for life. Primary infection may cause infectious mononucleosis, while subsequent viral persistence and intermittent reactivations in the immunocompetent host do not exhibit clinical symptoms [Amon and Farrell, 2005]. Despite this apparent quiescence, EBV's life cycle provides a constant challenge to the immune system, which becomes obvious as soon as the host's immunity is impaired and EBV-associated diseases occur [van Esser et al., 2001; Pattle and Farrell, 2006].
Over the last decades, a number of experiments have been performed that allowed a closer examination of EBV's replication in vivo. Besides serology [Hornef et al., 1995; Obel et al., 1996; Schaade et al., 2001], cord-blood transformation assays of throat washings [Preiksaitis et al., 1992], spontaneous LCL outgrowth in peripheral blood mononuclear cells [PBMCs, Yao et al., 1985], and quantitation of viral DNA in saliva specimen [Obel et al., 1996; Payne et al., 1999; Ikuta et al., 2000] have been used to that end. Moreover, ex vivo analyses of tonsils from EBV seropositive individuals have been carried out [Hislop et al., 2005; Hudnall et al., 2005]. From experiments using these techniques, it became evident that viral replication occurs intermittently at some permissive sites (i.e., the tonsils) and that symptomless viral reactivation from latency seems to be part of EBV's life cycle [Ikeda et al., 2000; Laichalk and Thorley-Lawson, 2005]. A conclusive picture of why these events take place and at which frequency they occur has not yet been defined, which is in part due to the limited sensitivity and/or low timely resolution of the experimental methods mentioned above, as well as their laborious and time-consuming nature. Detection of EBV-specific IgG, IgM, and IgA antibodies using enzyme-linked immunosorbent assays (ELISA), in particular, is known to underestimate the frequency of EBV reactivations in healthy carriers [Maurmann et al., 2003]. Moreover, antibody responses in general are not coincident with the actual event and elevated titers may persist well beyond clearance of the virological challenge.
Recent studies took advantage of quantitative real-time PCR in order to characterize EBV's life cycle more closely [Jabs et al., 2001; Wagner et al., 2002; Maurmann et al., 2003]. Herein, it was argued that reactivation of latently infected memory B cells by differentiation into plasma cells would be followed by a short period of viremia and consecutive re-infection of naïve B cells. Detection of plasma viremia and increases in viral load in PBMCs (as EBV genome equivalents per µl plasma and µg DNA, respectively) should therefore reflect directly viral replication. While it seems that EBV is replicating continuously in the tonsils of healthy carriers [Hudnall et al., 2005], replication does not occur usually in the peripheral blood. Earlier observations of detectable plasma viremia and increases in viral load in PBMCs even in healthy carriers [1–4 events per year, Maurmann et al., 2003] therefore were the basis to speculate that EBV's persistence is controlled tightly by the EBV-specific T-cell immune system and that minor disturbances of EBV-specific immunity are responsible for detectable viral replication in the peripheral blood. This hypothesis is supported by data from immunosuppressed transplant recipients, in whom EBV DNA is found far more often than in healthy control groups, which might be due to their strongly impaired T-cell function [Wagner et al., 2002; Leung et al., 2004].
In order to investigate further the relationship between EBV reactivations in the peripheral blood and EBV-specific T-cell immunity, a longitudinal observational study was performed analyzing peripheral T-cell frequencies by HLA tetramer-aided flow cytometry and lymphoblastoid cell line (LCL) and EBV peptide-specific ELISPOT assays. The results were compared to EBV plasma viremia and viral load in PBMCs as measures of EBV reactivation. It should be stressed that the definition of EBV reactivation used in this article is based solely on virological, but not on any clinical data or symptoms, and that the term “reactivation” relates to detectable plasma viremia or obvious increases in viral load.
MATERIALS AND METHODS
Study Population
Fourteen healthy, EBV seropositive individuals (for details see Table I) volunteered to donate whole blood samples at irregular intervals over a period of about 1 year. The study participants' health was screened by a physician before each sample was drawn in accordance with the German guidelines for blood donation. EDTA-anticoagulated blood was centrifuged immediately at 600 × g for 10 min and plasma was stored at −80°C until further use. PBMCs were isolated by standard density separation (Ficoll separation solution by Biochrom, Berlin, Germany) from lithium heparin-anticoagulated blood, and an ELISPOT assay was performed on the same day. Also, PBMCs were kept frozen at both −80 and −196°C for later DNA isolation and flow cytometric analysis, respectively. PBMCs were resuspended in 200 µl phosphate-buffered saline (PBS) after washing and then frozen immediately at −80°C. PBMCs to be kept at approximately −196°C were resuspended gently in 500 µl serum obtained from AB-positive blood donors at the local blood bank (Institute for Immunology and Transfusion Medicine, Luebeck School of Medicine, Germany) before adding a total of 500 µl volume comprised of 100 µl DMSO (Sigma, Munich, Germany), 200 µl RPMI (PAA, Pasching, Austria), and 200 µl serum of AB-positive blood donors. To minimize cell damage, PBMCs thus prepared were frozen at approximately 1°C per hour in ethanol for 24 hr before storing them in liquid nitrogen at −196°C.
| No. | Gender | Age | HLA class I haplotype | EBV reactivation | IFN-γ ELISPOT | |
|---|---|---|---|---|---|---|
| LCL reactivity | EBV peptide reactivities | |||||
| 2 | Male | 57 | A3, B13/35 | Yes | ↓ | ↔ |
| 3 | Female | 26 | A3, B7/35 | Yes | ↓ | ↔ |
| 4 | Male | 40 | A1/24, B57 | Yes | ↓ | ↔ |
| 9 | Male | 39 | A2/24, B5/7 | Yes | ↓ | ↔ |
| 12 | Male | 35 | A1/3, B7/57 | Yes | ↓ | ↔ |
| 14 | Male | 32 | A26/31, B27/62 | Yes | ↓ | ↔ |
| 5 | Male | 35 | A1, B8/51 | Yes | ↓↑ | ↑ |
| 10 | Male | 34 | A2, B8/60 | Yes | ↓↑ | ↑ |
| 1 | Male | 31 | A3/11, B7/51 | Yes | ↔ | ↑ |
| 6 | Male | 35 | A2, B8/44 | Yes | ↔ | ↑ |
| 7 | Male | 35 | A2/31, B51/62 | Yes | ↔ | ↑ |
| 13 | Male | 61 | A29, B44 | Yes | ↔ | ↑ |
| 8 | Male | 41 | A2, B13/62 | No | ↓↑ | ↔ |
| 11 | Male | 64 | A2, B61 | No | ↔ | ↔ |
- ↓: Substantially decreased IFN-γ response at times of detectable viral activity. ↔: IFN-γ response unchanged throughout study period, ↑: increase in LCL or peptide epitope specific IFN-γ responses at times of EBV reactivation.
ELISPOT
Depending on class I HLA antigens of each donor, PBMCs reactivity against a set of established immunodominant CD8 EBV epitope peptides was screened, as well as reactivity against the autologous LCL (for the full list of epitope peptides and references see Table II). According to the instructions of the manufacturer (h-IFN-gamma ELISpot kit ALP, MABTECH AB, Hamburg, Germany), 96-well plates (Millipore, Schwalbach, Germany) were coated with 50 µl anti-human IFN-γ antibodies at 15 µg/ml (part of the kit) and incubated at 4°C over night. Plates were washed then with PBS four times and remaining free binding sites were blocked by incubation with RPMI1640 medium (PAA, Pasching, Austria) containing 10% fetal calf serum (PAN-Biotech, Aidenbach, Germany) for 1 hr. For each peptide and the autologous LCL, 250,000 as well as 62,500 cells, each in duplicates, were dispensed into the coated microplate wells. Ten microliter of the appropriate peptide solution (Thermo Electron, Ulm, Germany) containing 2 µg peptide with a final concentration of 20 µg/ml were added according to the plate layout. A total of 50,000 cells of the autologous LCL were added to the appropriate wells. Positive controls were stimulated with 1 µg/ml phytohemagglutinin (Sigma); background controls remained unstimulated. Finally, all wells were filled to 100 µl by adding cell culture medium. The assays were incubated at 37°C for 24 hr before washing six times with PBS. Fifty microliter biotinylated anti-hIFN-γ antibody were added then at a concentration of 1 µg/ml, and after two hours at room temperature plates were washed another six times with PBS. Streptavidin-conjugated alkaline phosphatase in 50 µl PBS was added to each well and incubated for one hour at room temperature, and plates were washed another eight times with PBS. The color reaction was developed finally using 100 µl 5-bromo-4-chloro-indolyl-phosphatase/nitroblue tetrazolium (BCIP/NBT) per well (Moss Inc., Pasadena, MD) until distinct spots emerged as suggested by the kit's manual.
| HLA | Protein | Epitope | References |
|---|---|---|---|
| A2 | BMLF1 | YVLDHLIVV |
Saulquin et al. [2000 ] |
| BMLF1 | GLCTLVAML* |
Steven et al. [1997 ] |
|
| gp85 | LMIIPLINV |
Khanna and Burrows [2000 ] |
|
| gp350 | VLQWASLAV |
Khanna et al. [1999 ] |
|
| gp110 | ILIYNGWYA* |
Khanna and Burrows [2000 ] |
|
| EBNA3A | SVRDRLARL* |
Burrows et al. [1994 ] |
|
| EBNA3C | LLDFVRFMGV |
Kerr et al., [1996 ] |
|
| LMP2A | LLWTLVVLL |
Lee et al. [1997 ] |
|
| LMP2A | CLGGLLTMV* |
Lee et al. [1993 ] |
|
| A3 | EBNA3A | RLRAEAQVK |
Hill et al. [1995a ] |
| BRLF1 | RVRAYTYSK |
Khanna and Burrows [2000 ] |
|
| A11 | EBNA3B | IVTDFSVIK |
Gavioli et al. [1993 ] |
| EBNA3B | LPGPQVTAVLLHEES |
Gavioli et al. [1993 ] |
|
| LMP2A | SSCSSCPLSKI |
Lee et al. [1997 ] |
|
| A24 | BRLF1 | DYCNVLNKEF |
Khanna and Burrows [2000 ] |
| EBNA3A | RYSIFFDY |
Burrows et al. [1994 ] |
|
| EBNA3B | TYSAGIVQI |
Rickinson and Moss [1997 ] |
|
| LMP2A | TYGPFVMCL |
Lee et al. [1997 ] |
|
| A29 | EBNA3A | VFSDGRVAC |
Khanna and Burrows [2000 ] |
| B7 | EBNA3A | RPPIFIRRL |
Hill et al. [1995b ] |
| EBNA3C | QPRAPIRPI |
Hill et al. [1995b ] |
|
| B8 | EBNA3A | QAKWRLQTL* |
Burrows et al. [1994 ] |
| EBNA3A | FLRGRAYGL* |
Burrows et al. [1990a ] |
|
| BZLF1 | RAKFKQLL* |
Bogedain et al. [1995 ] |
|
| B27 | EBNA3B | RRARSLSAERY |
Rickinson and Moss [1997 ] |
| EBNA3C | LRGKWQRRYR |
Brooks et al. [1993 ] |
|
| EBNA3C | RRIYDLIEL |
Brooks et al. [1993 ] |
|
| EBNA3C | FRKAQIQGL |
Rickinson and Moss [1997 ] |
|
| LMP2A | RRRWRRLTV |
Khanna and Burrows [2000 ] |
|
| B35 | BALF4 | APGWLIWTY |
Khanna and Burrows [2000 ] |
| EBNA3A | YPLHEQHGM |
Burrows et al. [1994 ] |
|
| EBNA3B | AVLLHEESM |
Rickinson and Moss [1997 ] |
|
| B44 | EBNA3C | KEHVIQNAF |
Khanna et al. [1992 ] |
| EBNA3B | VEITPYKPTW |
Rickinson and Moss [1997 ] |
|
| EBNA3C | EGGVGWRHW |
Morgan et al. [1996 ] |
|
| EBNA3C | EENLLDFVRF |
Burrows et al. [1990b ] |
|
| B60 | LMP2A | IEDPPFNSL |
Lee et al. [1997 ] |
| B62 | EBNA3A | LEKARGSTY |
Rickinson and Moss [1997 ] |
| EBNA3B | GQGGSPTAM |
Rickinson and Moss [1997 ] |
- Epitopes for which HLA tetramers were available are marked with an asterisk (GLC, ILI, SVR, CLG, QAK, FLR, and RAK).
Analysis of the assay was carried out on an automatic spot analyzer (Elispot Reader System ELR03, Autoimmun Diagnostika, Straßberg, Germany) using the same software (Elispot 3.2.2, Autoimmun Diagnostika) and settings during the whole experiment. The absolute number of spots was determined for each well, and unspecific background activity, determined from the unstimulated negative controls, was subtracted always. As long as the absolute number of spots did not exceed 200 in each of the 250,000 cell assays, the mean value of both these wells was used to calculate the amount of spot forming units (sfu) per 1,000,000 cells. Otherwise, the mean value of both wells containing 62,500 cells was used for calculation in order to ensure accuracy of the assay if a large number of PBMCs showed EBV-specific reactivity. If well saturation, as determined by the ELISPOT reader software, exceeded 60% or the absolute number of spots in those wells containing 62,500 cells was >200, the well's final result was arbitrarily set to 5,000 sfu per 1,000,000 cells to point out obvious peaks in the final analysis.
Serology
EBV serology was determined by quantitation of EBV-specific IgG, IgM, and IgA antibodies using commercially available ELISA kits (Enzygnost, Dade Behring, Marburg, Germany) that employ a mixture of both latent and lytic viral antigens. Plasma samples were pre-absorbed with anti-IgG antibodies prior to analysis of IgM and IgA. According to the manufacturer, a final optical density (OD) of >0.120 determined IgM seropositivity. The corresponding value for IgA was >0.600, and for IgG >0.200. IgG antibodies were quantified using the alpha-method as recommended by the manufacturer, and results were expressed as international units per ml. Reactivation was considered when either IgM values were positive in the presence of detectable IgG, or when, coincident with positive IgA values, IgG exceeded 650 IU/ml.
Lymphoblastoid Cell Lines (LCL)
Lymphoblastoid cell lines were established from each donor before outset of the study. To this end, PBMCs were washed and resuspended in 4 ml supernatant of PMA-stimulated B95-8 cells; 100 µg/ml cyclosporine A were added 2 hr later. After 24 hr, PBMCs were cultured at 37°C in cell culture medium containing cyclosporine A for 3 weeks before standard cell culture medium was used and substituted for fresh medium on a regular basis.
Real-Time PCR
DNA isolation of PBMCs and plasma was carried out using the QIAmp DNA Blood Mini Kit (Qiagen, Hilden, Germany). PBMCs and plasma specimen were thawed by incubation at 37°C for 5 min. 5 × 106 of these PBMCs and 200 µl plasma, respectively, were used to isolate DNA according to the manual that was supplied with the kit. A TaqMan 7500 real-time PCR System (Applied Biosystems, Darmstadt, Germany) was used; thermal cycler conditions and assay preparation as well as primer/probe design and quantitation by normalization against a co-amplified control have been described before in detail [Jabs et al., 2001; Maurmann et al., 2003].
HLA Tetramer Synthesis
Synthesis of HLA tetramers was described elsewhere [Altman et al., 1996]. Briefly, BL21-DE3 and BL21-DE3pLysS Escherichia coli (Invitrogen, Karlsruhe, Germany) were transformed with β2 microglobulin and HLA-A2 and -B8 DNA, respectively. Protein expression of β2 microglobulin and the heavy chains was induced by IPTG (isopropyl β-D-1-thiogalactipyranoside), and protein was harvested by ultrasound treatment and solubilization with urea. Appropriate HLA-A2 and -B8 EBV-specific epitope peptides (Table II), β2 microglobulin and the corresponding heavy chain were brought together for refolding to monomers before concentration of the obtained solution. The monomers were biotinylated by means of the BirA enzyme. Gel filtration was used to separate HLA class I complexes from debris according to their size, and ion exchange served for further purification and removal of non-biotinylated monomers. Both protein assay and biotinylization assay took place before the final step of making tetramers by adding PE-labeled streptavidin to the monomers.
Flow Cytometry
Deep-frozen PBMCs were thawed by incubation at 37°C for 5 min and washed by addition of 5 ml PBS containing 2% fetal calf serum (FACS buffer) and centrifugation at 300 × g for 10 min. The pellet was resuspended in FACS buffer and aliquoted to flow cytometry tubes depending on how many different HLA tetramers were available for each donor. Five microliter of PE-labeled HLA tetramers were added to each sample before incubation at 37°C for 15 min. Cells were washed in 4°C cold FACS buffer, and incubated for 25 min at room temperature with 5 µl tricolor-labeled CD8 antibody (Invitrogen; isotype IgG2a, clone 3B5). After washing in FACS buffer, cells were resuspended in 250 µl PBS containing 2% paraformaldehyde (Sigma).
Flow cytometry was performed on Cytomics FC500 (Beckman Coulter, Krefeld, Germany). Before each measurement, compensation was done using cells from the sample that underwent exactly the same procedures as the others, but were not stained with HLA-tetramers: (i) cells that remained completely unstained, (ii) cells that were stained with PE-labeled CD8 antibodies (Becton Dickinson, Heidelberg, Germany; isotype mouse IgG1, clone RPAT8), and (iii) cells that were stained with tricolor-labeled CD8 antibodies. Analysis of raw data was aided by FlowJo 7.1 software (TreeStar, Inc., Ashland, OR). The measurements were aimed at counting at least 750,000 cells. Gating was performed using forward and side scatter to determine the viable lymphocyte population. The CD8+ lymphocyte subset was quantified and gated using a histogram plot, from which the number of tetramer positive cells was derived. The amount of tetramer positive cells is expressed as percentage of tetramer positive cells in the CD8+ lymphocyte gate (Fig. 1).
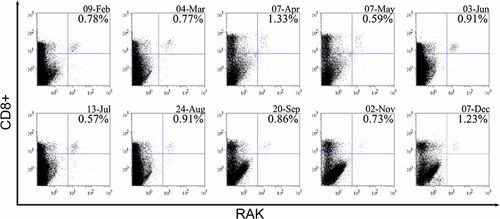
Examples of HLARAK-specific CD8+ T cells enumerated by flow cytometry. Flow cytometry results obtained from HLA-B8-positive donor no. 5 on respective dates (for corresponding ELISPOT data see Table III and Fig. 5a). PBMCs were stained with CD8 antibodies and RAK-specific HLA-tetramers. The amount of CD8+ RAK+ T lymphocytes is expressed as percentage of CD8+ T lymphocytes.
RESULTS
EBV Reactivations Among the Study Population
Using quantitative real-time PCR, plasma viremia and/or significant changes in viral load in PBMCs was detected in 12 out of 14 study participants, which accounts for 86%. Detectable plasma viremia was found in nine individuals, significant changes in viral load were observed in seven donors; thus, four participants displayed both entities at least once. Changes in viral load in PBMCs were defined as being significant as soon as obvious peak values exceeded a participant's mean viral load by at least three times the standard deviation or as soon as donors displayed detectable viral load in PBMCs only once.
Contrastingly, EBV serology failed mainly to detect viral reactivations (data not shown). Merely, one individual (donor no. 14) displayed an episode of detectable IgM titers in the presence of IgG antibodies, and this episode was accompanied by detectable plasma viremia. All remaining patients exhibited typical serology of past EBV infection with IgG seropositivity in the absence of IgM. Furthermore, none of the study participants had detectable IgA antibodies coincident with elevated IgG titers, which also would have been suggestive of EBV reactivation.
Frequency of EBV-Specific T Cells
Reactivity against autologous LCL
PBMCs reactivity against autologous EBV-transformed LCL was quantified by means of IFN-γ ELISPOT. Nine out of 14 study participants displayed remarkably variable courses of LCL-specific T-cell frequencies, eight of which had viral reactivation (Table I). The episodes of detectable viral reactivation occurred preferably at times of substantially diminished LCL-specific immunity, which is shown in detail for participant nos. 2, 3, 4, 9, 12, and 14 (Fig. 2a–f). However, decreased LCL-specific immunity was not necessarily a prerequisite for detectable viral activity, as donor nos. 5 and 10 displayed elevated as well as diminished IFN-γ responses during episodes of EBV reactivation (Fig. 3a,b). Moreover, diminished IFN-γ responses against autologous LCL did not result automatically in detectable viral activity as shown in Figure 2. Stable LCL-specific T-cell reactivities without signs of viral reactivation were observed only in participant no. 11, which is depicted in Figure 4 (donor no. 11).

a–f: Time courses of EBV reactivation and EBV-specific IFN-γ releasing T cells in donors with diminished LCL-specific T-cell reactivity during EBV reactivation. The diagram on top depicts virological markers determined by real-time PCR. The second chart shows the results of LCL- and peptide-stimulated ELISPOT assays expressed as spot forming units per one million cells; the grey overlay highlights phases of viral reactivation. a: Donor no. 2, (b) donor no. 3, (c) donor no. 4, (d) donor no. 9, (e) donor no. 12, (f) donor no. 14.
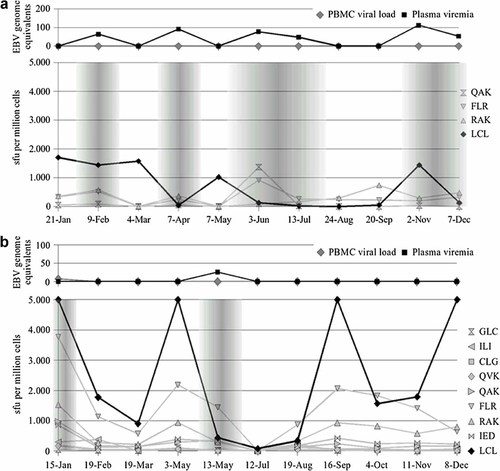
a,b: Time courses of EBV reactivation and EBV-specific IFN-γ releasing T cells in donors with fluctuating LCL-specific T-cell reactivity as well as increases in single epitope T-cell frequencies during EBV reactivation. The diagram on top depicts virological markers determined by real-time PCR. The second chart shows the results of LCL- and peptide-stimulated ELISPOT assays expressed as spot forming units per one million cells; the grey overlay highlights phases of viral reactivation. a: Donor no. 5, (b) donor no. 10.
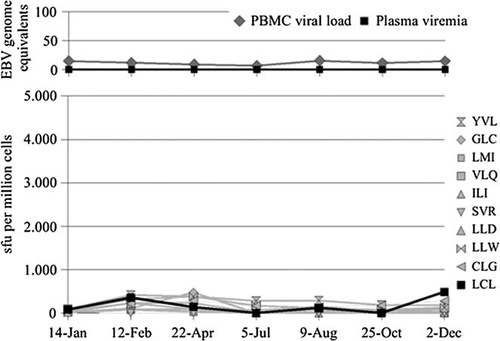
Time course of EB viral load and plasma viremia as well as EBV-specific IFN-γ releasing T cells in donor no. 11 without signs of viral reactivation. The diagram on top depicts virological markers determined by real-time PCR. The second chart shows the results of LCL- and peptide-stimulated ELISPOT assays expressed as spot forming units per one million cells; the grey overlay highlights phases of viral reactivation.
Positive control wells yielded always values >250 sfu per million cells with a majority >1,000 sfu per million cells. Importantly, positive controls did not imitate the course of LCL-specific responses at all, which minimizes the possibility of unspecific T-cell reactivity as a reason for fluctuating LCL responses (data not shown).
Epitope-specific T cells
In addition to the quantitation of LCL-specific T-cell frequencies by ELISPOT, PBMCs were stimulated with a set of known HLA class I restricted epitope peptides to determine epitope-specific reactivities—for reference, see Table II. The reactivity of these epitope-specific CD8+ T cells was less fluctuating compared to EBV-LCL stimulated PBMCs. Six out of 14 study participants displayed substantial increases in their T-cell reactivities against at least one peptide, as specified in Table I. As opposed to LCL reactivity, increases in single CD8 T-cell reactivities occurred almost exclusively concomitantly with viral reactivation (donor no. 1: epitope peptide RLR, donor no. 7: CLG, donor no. 13: EGG; Fig. 5a–c, and donor no. 5: QAK, FLR, Fig. 3a).
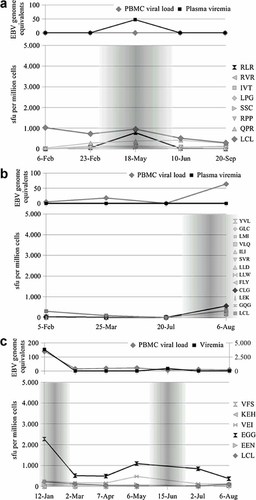
a–c: Time courses of EBV reactivation and EBV-specific IFN-γ releasing T cells in donors with stable LCL-specific T-cell reactivity but increased frequencies of single epitope-specific T cells during EBV reactivation. The diagram on top depicts virological markers determined by real-time PCR. The second chart shows the results of LCL- and peptide-stimulated ELISPOT assays expressed as spot forming units per one million cells; the grey overlay highlights phases of viral reactivation. a: Donor no. 1, (b) donor no. 7, (c) donor no. 13.
In addition to quantifying T-cell reactivities by IFN-γ ELISPOT, HLA tetramers (Table II) were employed to enumerate directly EBV-specific CD8+ T lymphocytes by flow cytometry in seven HLA-A2 and/or -B8 positive study participants. Due to limited availability of cell material only RAK, FLR, GLC, and CLG tetramers could be analyzed. The numbers of CD8+ T lymphocytes that stained with the mentioned tetramers also varied over time. However, no correlation was found between changes in epitope-specific T-cell numbers suggested by ELISPOT and those measured by flow cytometry (Table III). No association was observed between changing numbers of tetramer-stained CD8+ T cells and viral reactivation.
| Donor no. 5 | 09 February | 04 March | 07 April | 07 May | 03 June | 13 July | 24 August | 20 September | 02 November | 07 December | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HLAtet-RAK | 1,765 | 1,017 | 1,419 | 1,478 | 2,157 | 1,151 | 1,549 | 1,255 | 1,525 | 1,562 | |
| ELISPOT-RAK | 578 | 0 | 368 | 0 | 83 | 163 | 294 | 738 | 292 | 481 | |
| HLAtet-FLR | 22 | 860 | 1,939 | 1,716 | 2,321 | 1,062 | 985 | 1,043 | 745 | 826 | |
| ELISPOT-FLR | 516 | 0 | 245 | 0 | 913 | 262 | 244 | 224 | 200 | 325 |
| Donor no. 6 | 12 January | 09 February | 08 March | 05 May | 17 June | 23 August | 20 October | 20 December | |
|---|---|---|---|---|---|---|---|---|---|
| HLAtet-GLC | n.a. | 2,580 | 1,949 | 870 | 908 | 320 | 187 | n.a. | |
| ELISPOT-GLC | 310 | 341 | 205 | 245 | 488 | 333 | 207 | 107 | |
| HLAtet-CLG | 1,526 | 407 | 2,149 | 393 | 72 | 208 | 0 | n.a. | |
| ELISPOT-CLG | 252 | 361 | 277 | 315 | 704 | 520 | 279 | 315 | |
| HLAtet-RAK | 6,861 | 7,602 | 7,447 | 5,346 | 7,350 | 5,324 | 4,717 | n.a. | |
| ELISPOT-RAK | 1,044 | 1,725 | 1,505 | 1,478 | 1,840 | 1,495 | 1,703 | 1,043 | |
| HLAtet-FLR | 1,272 | 2,094 | 1,870 | 1,095 | 1,836 | 1,405 | 999 | n.a. | |
| ELISPOT-FLR | 380 | 861 | 1,313 | 450 | 590 | 636 | 607 | 469 |
| Donor no. 7 | 05 February | 25 March | 20 July | 06 August | |
|---|---|---|---|---|---|
| HLAtet-GLC | 866 | 534 | 1,496 | 232 | |
| ELISPOT-GLC | 45 | 0 | 0 | 264 | |
| HLAtet-CLG | 898 | 1,069 | 570 | 557 | |
| ELISPOT-CLG | 31 | 11 | 0 | 560 |
| Donor no. 8 | 03 May | 06 July | 13 September | 20 October | 09 December | |
|---|---|---|---|---|---|---|
| HLAtet-GLC | 1,487 | 541 | 1,538 | 913 | 908 | |
| ELISPOT-GLC | 62 | 203 | 400 | 265 | 103 | |
| HLAtet-CLG | 45 | 126 | 158 | 225 | 92 | |
| ELISPOT-CLG | 188 | 51 | 83 | 7 | 27 |
| Donor no. 9 | 28 January | 08 March | 28 April | |
|---|---|---|---|---|
| HLAtet-GLC | 3,536 | 1,629 | 1,683 | |
| ELISPOT-GLC | 140 | 91 | 79 | |
| HLAtet-CLG | 2,048 | 1,266 | 851 | |
| ELISPOT-CLG | 118 | 107 | 147 |
| Donor no. 10 | 19 February | 19 March | 03 May | 13 May | 12 July | 16 September | 08 December | |
|---|---|---|---|---|---|---|---|---|
| HLAtet-GLC | 300 | 1,711 | 952 | 374 | 351 | 361 | 1,334 | |
| ELISPOT-GLC | 19 | 17 | 99 | 21 | 0 | 18 | 25 | |
| HLAtet-CLG | 107 | 687 | 410 | 315 | 433 | 284 | 707 | |
| ELISPOT-CLG | 47 | 40 | 42 | 49 | 0 | 66 | 57 | |
| HLAtet-RAK | 2,630 | 6,604 | 3,284 | 2,172 | 1,410 | 1,518 | 2,870 | |
| ELISPOT-RAK | 270 | 210 | 935 | 293 | 0 | 926 | 796 | |
| HLAtet-FLR | 4,064 | 130 | 347 | 3,955 | 2,501 | 2,187 | 4,891 | |
| ELISPOT-FLR | 1,130 | 568 | 2,183 | 1,441 | 0 | 2,062 | 636 |
| Donor no. 11 | 14 January | 22 April | 05 July | 09 August | 25 October | 02 December | |
|---|---|---|---|---|---|---|---|
| HLAtet-GLC | 4,988 | 570 | 640 | 963 | 1,195 | 4,578 | |
| ELISPOT-GLC | 19 | 477 | 0 | 27 | 62 | 27 | |
| HLAtet-CLG | n.a. | 532 | 18 | 0 | 422 | 2,787 | |
| ELISPOT-CLG | 53 | 285 | 167 | 143 | 180 | 281 |
-
ELISPOT results are given in sfu per million cells, and flow cytometry data are shown as number of tetramer positive cells per 106 lymphocytes. Bold values indicate obvious increases in either sfu per million cells or in HLA
 cells.
cells.
DISCUSSION
The present study shows that cellular immunity against EBV is not a static phenomenon but that most virus carriers display a high degree of variability in their EBV-specific immune response. As mentioned before, it was hypothesized that times of weakened EBV-specific immunity might facilitate intermittent EBV reactivation and that in response these episodes might lead to an expansion of EBV-specific T cells, similar to the boostering of humoral antigen-specific immune responses after vaccination [Annels et al., 2006]. In fact, viral reactivations in the study population occurred preferentially at times of diminished LCL-specific immune responses, which support the first part of our hypothesis. Analysis of immunity against individual HLA class I restricted EBV-derived epitopes, however, provided different results: some epitope-specific T-cell frequencies were found to be increased during episodes of viral reactivation, which might reflect the second part of our hypothesis. The contradiction between decreased LCL-specific immune responses on the one and elevated epitope-specific cytotoxic T-cell reactivity on the other hand, might suggest different kinetics of CD8+ and CD4+ T lymphocyte as well as natural killer (NK) cell activity in controlling EBV reactivation (see below).
Results obtained by HLA-tetramer staining and flow cytometry in HLA-A2 and/or -B8 positive study participants showed that the frequency of EBV-specific CD8+ T lymphocytes varied over time, as well. However, no association could be observed to IFN-γ reactivity of peptide-specific CD8+ clones—as enumerated by ELISPOT analysis. Despite the small population examined by flow cytometry, the striking discrepancy between ELISPOT and FACS measurements needs further explanation. Flow cytometry is an outstanding method in determining cell population phenotypes on a single-cell level independent of their functional state [McCurley and Larson, 1996]. Usefulness and accuracy in quantifying antigen-specific lymphocytes have been demonstrated in countless publications [Spiegel et al., 2000; Gratama et al., 2001; Terajima et al., 2003]. Even though FACS allows for the detection of indirect markers of cell activation, for example, by intracytoplasmatic cytokine staining [Arora, 2002; Letsch and Scheibenbogen, 2003], ELISPOT provides a much more elegant way of assessing a cell population's functional capabilities: it visualizes cytokine secretion after stimulation and thereby might represent “immunity” as a reaction to antigen more accurately than the static and non-functional flow cytometry experiments. Thus, it might be possible that the frequencies of EBV epitope-specific T cells remain largely constant, but that their functional capacity varies as suggested by fluctuating IFN-γ release [Hess et al., 2004]. This could be explained by changing phenotypes induced by viral reactivation itself or by unspecific bystander activation [Doisne et al., 2004]. Further experiments are warranted to characterize phenotypes that are associated with EBV reactivation and/or changes in IFN-γ release. Due to limited cell numbers, these experiments could not be performed in the present study. In addition, it cannot be ruled out that the absolute numbers of peripheral lymphocytes or their subsets varied by instance or in response to viral reactivation and that those changes in numbers of peripheral lymphocyte accounted for some discrepancies observed in this study.
Another unexpected finding was that the sum epitope-specific T-cell frequencies in ELISPOT did not account for the reactivity against autologous LCL, which poses the question what the nature of PBMCs reactivity against LCL in ELISPOT might be. Research on EB viral immunity has long been dominated by investigation of CD8+ T cells, which are known for their direct cytotoxic effects, in both acute and chronic infection [Murray et al., 1992; Steven et al., 1997]. Their frequency and phenotype have been assessed, especially since appropriate HLA-tetramers have become available [Hislop et al., 2002]. CD4+ T cells, on the other hand, have been neglected somewhat and only in recent years have become a focus of attention, which, at least in part, is due to progress made in HLA class II tetramer synthesis and the discovery of HLA class II restricted viral epitopes [Amyes et al., 2003; Ye et al., 2004; Milosevic et al., 2006]. An increasing number of papers dealing with CD4+ T-cell cytotoxic capabilities in the context of EBV reflect the potential importance of these so-called T helper cells in controlling EBV infection and persistence [Adhikary et al., 2006; Heller et al., 2006]. Since PBMCs were used for ELISPOT without CD4+ and/or CD8+ cell depletion, LCL reactivity cannot be attributed to one of these populations. Sole reactivity against HLA class I restricted epitope peptides were determined, thereby excluding CD4+ cells. For these reasons, it is supposed that CD4+ T cells might play a dominant role in controlling persistent EBV infection [Gudgeon et al., 2005]. CD8+ T lymphocytes, however, are likely to have been overestimated in their representation of EB viral immunity as a whole, and further research is necessary to understand CD4+ T cells' contribution to maintaining the virus-host balance. In addition, NK cells may play a distinct role in regression of LCL outgrowth in vitro as well as in resolution of IM in vivo [Rooney et al., 1985; Tomkinson et al., 1989]. Although their in vivo function of controlling latent EBV infection is characterized poorly, it is possible that expansion of IFN-γ secreting NK cells accounted for some LCL reactivity in ELISPOT analysis with low epitope-specific T-cell frequencies. Second, it is possible that the number of lytically infected cells in lymphoblastoid cell lines, and thus the cell line's antigenicity, varies over time. As this has never been investigated before, it cannot be ruled out that considerable fluctuation in LCL-specific T-cell frequencies are due to changes occurring naturally in lymphoblastoid cell lines [Staege et al., 2002]. This would explain why in some cases, LCL-specific T cells were outnumbered by T cells specific for merely one viral epitope in ELISPOT. Third, although the possible importance of CD4+ T cells regarding EBV surveillance in vivo has been pointed out before, it is conceivable that yet unknown HLA class I restricted epitopes and pertaining CD8+ T lymphocytes might play a major role in controlling persistent EBV infection in vivo. The present study employed a set of well-known latent and lytic HLA class I restricted epitopes to monitor the participants' T-cell reactivities, which were thought to cover the bulk of EBV-specific immunity. With 14 different individuals and the variety of HLA molecules they display, the existence of so-far unknown immunodominant epitopes seems feasible, of course, although the sheer discrepancy between the sum of peptide reactivities and LCL reactivities renders this possibility as the only explanation unlikely.
Since persistence of EBV is controlled tightly by the host's immune system, phases of viral replication, viremia and consecutive increases in infected B-cell numbers, are thought to be of fairly short duration. The study design unfortunately did not allow access to participating blood donors on a weekly or biweekly basis, which would have provided a more accurate picture of virological parameters for the diagnosis of EBV reactivation. In addition, the study population consisted almost exclusively of men which might be significant, when interpreting the data. Moreover, the total number of lymphocytes was not taken into account, when interpreting the ELISPOT data with respect to virus reactivation. It cannot be ruled out that the level of immunity changed solely by intraindividual changing of lymphocyte numbers. Given these limitations, it must be assumed that the number of symptomless episodes of viremia and increases in viral load in PBMCs is larger than detected, and that in many cases of diminished LCL-specific immunity, where no signs of viral activity have been observed, infected B cells and virions in the blood already might have been purged by the study participant's immune system before examination.
In summary, the present study shows that immunity against EBV varies considerably over time in most individuals. It is concluded that diminished EBV-specific LCL reactivity might facilitate intermittent viral reactivation. However, flow cytometry employing HLA tetramers showed that EBV-specific CD8+ T lymphocyte numbers do not increase or decrease during episodes of viral reactivation, which might suggest changes in phenotype and reactivity rather than clonal expansion of EBV-specific T cells.
Acknowledgements
We are indebted to the generous help of Prof. Alan B. Rickinson and his T-cell group, namely Nancy Gudgeon, Graham Taylor, and Andrew Hislop, at CR-UK Institute for Cancer Studies, University of Birmingham, UK.




