Intrathecal antibody production in two cases of yellow fever vaccine associated neurotropic disease in Argentina
Abstract
During the period 2007–2008 several epizootics of Yellow fever with dead of monkeys occurred in southeastern Brasil, Paraguay, and northeastern Argentina. In 2008 after a Yellow fever outbreak an exhaustive prevention campaign took place in Argentina using 17D live attenuated Yellow fever vaccine. This vaccine is considered one of the safest live virus vaccines, although serious adverse reactions may occur after vaccination, and vaccine-associated neurotropic disease are reported rarely. The aim of this study was to confirm two serious adverse events associated to Yellow fever vaccine in Argentina, and to describe the analysis performed to assess the origin of specific IgM against Yellow fever virus (YFV) in cerebrospinal fluid (CSF). Both cases coincided with the Yellow fever vaccine-associated neurotropic disease case definition, being clinical diagnosis longitudinal myelitis (case 1) and meningoencephalitis (case 2). Specific YFV antibodies were detected in CSF and serum samples in both cases by IgM antibody-capture ELISA. No other cause of neurological disease was identified. In order to obtain a conclusive diagnosis of central nervous system (CNS) infection the IgM antibody index (AIIgM) was calculated. High AIIgM values were found in both cases indicating intrathecal production of antibodies and, therefore, CNS post-vaccinal YFV infection could be definitively associated to YFV vaccination. J. Med. Virol. 83:2208–2212, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Yellow fever virus (YFV), from the Flavivirus genus, family Flaviviridae, causes an acute viral hemorrhagic disease, known as Yellow fever. Yellow fever is transmitted through two major cycles, the sylvatic and the urban. This last is transmitted from human to human by the domestic mosquito Aedes aegypti [Monath, 1994].
Yellow fever remains an important public health problem in the regions of Africa and South America where periodic epidemics have been produced since 1647 [Bres, 1986; Monath, 2001]. The World Health Organization (WHO) estimates that YFV causes 200,000 cases of clinical disease and 30,000 deaths each year [Staples et al., 2010]. In South America, transmission of YFV occurs predominantly in sparsely populated forested areas. Since 2007 an expansion of the endemic zone to southern and eastern areas has been observed with several epizootics, monkey deaths, caused by YFV in southeastern Brasil and northeastern Argentina [PAHO, 2009; Cardoso Jda et al., 2010]. In Argentina the epizootics affected Misiones and Corrientes provinces [PAHO, 2009]. In 2008 a Yellow fever outbreak caused seven human cases in Misiones province [COFESA, 2008].
In the absence of an effective antiviral treatment, prevention by vaccination reduced greatly morbidity and mortality associated with Yellow fever. Historically, the 17D live attenuated YFV vaccine has been considered to be one of the safest and most effective live virus vaccines [WHO, 2008]. Mild side effects occur in 15–20% of vaccinees after administration of Yellow fever vaccine including headache, myalgia, and fever. Serious adverse effects have been reported rarely (4.75 per 100,000 doses) [Martin et al., 2001; Vasconcelos et al., 2001; Staples et al., 2010]. Three different types of serious adverse reactions may occur after vaccination: (i) hypersensitivity reactions such as allergy, predominantly in persons with a history of allergy to eggs [Martin et al., 2001; Vasconcelos et al., 2001; Staples et al., 2010]; (ii) Yellow fever vaccine-associated viscerotropic disease reported as febrile multiple organ system failure [Martin et al., 2001; Vasconcelos et al., 2001]; and (iii) Yellow fever vaccine-associated neurotropic disease represented as a conglomerate of different clinical syndromes, including meningoencephalitis, Guillain–Barré syndrome, acute disseminated encephalomyelitis, bulbar palsy, and Bell's palsy [Jennings et al., 1994; WHO, 2008]. Nevertheless, these neurological reactions are extremely uncommon, rarely fatal, and have been reported in persons of all ages. The onset of illness for documented cases ranges 1–30 days after vaccination, and almost all cases were in first-time vaccine recipients [WHO, 2008].
One of the elements required for the confirmation of a suspected Yellow fever vaccine-associated neurotropic disease case is the detection in cerebrospinal fluid (CSF) of IgM antibodies specific to YFV [WHO, 2008]. Serological diagnosis of central nervous system (CNS) infection requires the proof of intrathecal synthesis of specific antibodies. However the antibodies may be passively transferred from serum to CSF reflecting a lesion in the blood–brain barrier [Deibel and Schryver, 1976; Reiber, 1994]. In order to obtain a conclusive diagnosis of CNS infection the IgM antibody index should be calculated.
After the outbreak of YFV in South America in 2007/2008 and as part of an exhaustive prevention campaign 1,503,556 Yellow fever vaccine doses were distributed between January and April 2008 in Argentina. Eighteen mild adverse events and only one case of serious adverse event, Yellow fever vaccine-associated neurotropic disease, were reported [COFESA, 2008]. The aim of this study was to confirm two other serious adverse events associated to Yellow fever vaccine in Argentina, and to describe the analysis performed to assess the origin of specific IgM against YFV in CSF.
MATERIALS AND METHODS
Case-Samples
Given the Yellow fever vaccine-associated neurotropic disease suspicion, serum, and CSF samples were obtained from both patients during the course of disease. The samples were obtained on days 49th and 30th post-vaccination for case 1 and 2, respectively.
Isolation of RNA and Amplification
Total RNA was extracted from CSF and blood samples of both cases using Trizol (Invitrogen, Carlsbad, CA) and was purified by the RNAid kit (Bio 101, QBioGene, Solon, OH) following manufacturer´s recommendations. Single round and heminested reverse-transcription polymerase chain reaction (RT-PCR) procedures were performed in CSF samples to amplify two partial fragments from flavivirus genome using One Step RT-PCR kit (Qiagen GmbH, Hilden, Germany) and Hot Start Taq DNA polimerase (Qiagen GmbH, Hilden, Germany), respectively, following the instruction of the manufacturer. The primers used in the single round RT-PCR were published previously as flavivirus universal primer pair [Tanaka, 1993]. This primer pair is located in the highly conserved non-structural protein 5 (NS5) and non-translated region of the genome. For the heminested RT-PCR reactions the primers used to amplify a partial fragment corresponding to the NS5 protein were cFD2, MAMD, and FS 778 [Scaramozzino et al., 2001]. SYBR Green real-time RT-PCR was also performed in CSF and blood samples of both cases. The cDNA was firstly obtained by reverse transcription reaction (Omniscript RT kit, Qiagen GmbH, Hilden, Germany) using cFD2 primer followed by real-time PCR with cFD2 and FS 778 primers using a MyiQ single color RT-PCR detection system (BioRad, Hercules, CA).
YFV-Specific IgM Capture Enzyme-Linked Immunosorbent Assay (ELISA)
To compare antibody levels in CSF and serum, both samples were tested simultaneously in the same test of YFV-specific IgM capture ELISA. Plates were coated with affinity-purified goat antihuman IgM (µ) antibody (Kierkegaard and Perry Laboratories. KPL. Baltimore, MD). All the following incubations were done at 37°C for 1 hr and the plates were washed six times in wash buffer (Tween-20 0.1% in PBS). After blocking, serial fourfold dilutions (from 1:100) of serum sample and serial twofold dilutions (from 1:5) of CSF sample were allowed to react with the antihuman IgM (µ) antibody-coated wells. Inactivated sucrose–acetone extracted Yellow fever antigen and control antigen at optimal dilutions were added to each well. Positive samples were detected by incubation firstly with rabbit polyclonal serum to YFV and then with goat anti-rabbit IgG conjugated with horseradish peroxidase and ABTS (2,2′-azino-bis [3-ethylbenthiazoline-6-sulfonic acid] substrate, Kirkegaard and Perry Laboratories). Absorbance was read at 405 nm (A405). Since the antibodies against YFV and Dengue virus are cross-reactive and both flaviviruses circulate in Argentina, an IgM capture ELISA to Dengue virus was performed simultaneously using the same protocol replacing the antigen by inactivated sucrose–acetone extracted Dengue virus antigen and the rabbit polyclonal serum to YFV by serum to Dengue virus.
Yellow Fever Vaccine-Associated Neurotropic Disease Case Definition
A suspected case of Yellow fever vaccine-associated neurotropic disease is defined as a person that develop one or more of the following symptoms within 1–30 days after vaccination: fever, headache, focal neurological dysfunction, change in mental status, CSF pleocytosis (≥5 leucocytes/mm3), and/or elevated CSF protein (>1.5 times the normal level), and also complemented by neuroimaging consistent with inflammation or electroencephalogram finding consistent with encephalopathy; no evidence of other diagnosis must be present [WHO, 2008]. A suspected case of Yellow fever vaccine-associated neurotropic disease become confirmed if at least one of the following elements is present: detection in CSF of IgM antibodies specific to YFV, or isolation of Yellow fever 17D viral vaccine strain from CSF or amplification of 17D viral strain from CSF [WHO, 2008].
Intrathecal Synthesis
-
Qalb = albuminCSF/albuminserum, measured by nephelometry.
-
AIIgM = QIgM-specific/QIgM-lím.
-
QIgM-lím = 0.67 × [
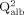 + (120 × 10−6)]½ − 7.1 × 10−3 is the limiting IgM quotient.
+ (120 × 10−6)]½ − 7.1 × 10−3 is the limiting IgM quotient. -
QIgM-specific = AUCSF/AUserum is the ratio of YFV-specific IgM in CSF and serum expressed in arbitrary units (AU).
Absorbance of serum and CSF specimen were converted to AU in a log–log diagram based on a standard curve derived from 7 twofold serial dilutions of a positive serum pool with A405 values between 0.08 and 2.6; the highest standard concentration was defined as 100 AU [Reiber and Lange, 1991; Tumani et al., 1995]. An AIIgM value greater than 1.4 was considered a positive result for intrathecal IgM synthesis as a criterion of CNS involvement [Reiber and Lange, 1991; Reiber, 1994].
RESULTS
Clinical Presentation
Case 1 was a healthy 53-year old man who stated that to have traveled to an endemic Yellow fever zone in South America after receiving the 17D vaccine. Clinical presentation has been previously described [Chaves et al., 2009]. Briefly, after 45 days post-vaccination symptoms began with headache, fever, and malaise. He presented progressive paraparesia, urinary retention, and constipation that began 48 hr previous to the admission. On examination, the patient had symmetric proximal weakness of the lower extremities. Other systemic examinations showed normal. Lumbar puncture was performed 49 days post-vaccination. Symptoms began to improve 5 days after admission. Subsequently, the patient needed physical rehabilitation with significant improvement in the strength of his lower limbs.
Case 2 was a 63-years-old previously healthy man who received Yellow fever vaccine together with tetanus and diphtheria vaccines. Four days later he developed fever, fatigue, myalgia, and evolved to confusional syndrome with episodes consistent of expression aphasia and mnestic disorders so he was admitted to a county hospital where a CSF analysis showed clear and colorless fluid with normal cell count, glucose, protein, and opening pressure. Thirty days after the symptoms began he was transferred to a third level hospital at the request of his family. Neurological examination revealed cerebellar syndrome (positive Romberg sign, adiadochokinesia) and disartria. The examination of the senses and cranial nerves were normal. Laboratory tests on admission displayed no relevant data but hiponatremia that responded to fluid intake restriction (inappropriate antidiuretic hormone production). The CSF showed normal protein and glucose levels with pleocytosis (39 leukocytes/mm3 with 75% lymphocytes). Brain magnetic resonance imaging showed diffuse periventricular hyperintensity. On day 37 of his illness, the patient showed improvement and was dismissed.
Serological and Genetic Diagnosis
Serological tests were negative for HIV, VDRL, and HTLV for both cases. Serum detection for acute cytomegalovirus, Epstein-Barr virus, chlamydia, and mycoplasma infections were also negative for case 1. In case 2, cryptococcal antigen detection was performed in CSF sample with negative result. Cultures done from CSF samples of both cases for enteroviruses, HSV, HHV6, and VZV were negative.
Both cases showed negative results in single round RT-PCR and heminested RT-PCR for the detection of YFV RNA in CSF samples. In order to confirm the molecular results obtained, a real-time RT-PCR was done in CSF and blood samples for the two cases, none of the samples analyzed exhibited positive signals.
Yellow Fever Vaccine-Associated Neurotropic Disease Case Confirmation
Yellow fever virus-specific IgM capture ELISA was positive in serum and CSF samples. In case 1 reciprocal values of the ELISA titer were 60 and 6400 in CSF and serum samples, respectively, and in case 2 were 20 and 400, respectively. Dengue virus antibodies were not detected.
Increased values of Qalb indicate dysfunction of blood–brain barrier because albumin originates exclusively from blood [Ganrot and Laurell, 1974; Reiber and Peter, 2001]. The results of this work would indicate blood–brain barrier damage only for case 1 according with the upper reference limit previously defined for the corresponding age group: 8 × 10−3 [Reiber and Peter, 2001] (Table I). However, in spite of this damage the high AIIgM value (>1.4) indicates intrathecal production of antibodies. For case 2, the blood–brain barrier was not disturbed, indicating that the YFV-specific IgM detected in CSF was locally synthesized (Table I).
| Case 1 | Case 2 | |
|---|---|---|
| Age | 53 | 63 |
| Clinical diagnosis | Longitudinal myelitis | Meningoencephalitis |
| YFV-specific IgM capture ELISAa | + | + |
| YFV real-time RT-PCRa | − | − |
| CSF (white blood cells/mm3) | 110 | 39 |
| CSF lymphocytes (%) | 90 | 75 |
| CSF protein concentration (mg/dl) | 56 | 34 |
| CSF albumin (mg/dl) | 44.8 | 27 |
| Serum albumin (g/dl) | 4 | 4.1 |
| Qalb | 11.2 × 10−3 | 6.6 × 10−3 |
| AIIgM | 21.19 | 41.2 |
- a Performed in serum and CSF samples.
DISCUSSION
Yellow fever vaccine-associated neurotropic disease was seen primarily among infants with encephalitis, but more recent reports describe this adverse event among persons of all ages [Staples et al., 2010]. The reported rate for Yellow fever vaccine-associated neurotropic disease is 0.4–0.8 cases per 100,000 doses distributed and is higher in persons older than 60 years of age [Staples et al., 2010].
Serological diagnosis of CNS infection requires proof of intrathecal synthesis of specific antibodies. The Al discriminates between a blood-derived and a pathological brain-derived specific antibody fraction in CSF and takes into account individual changes in blood/CSF barrier function [Reiber and Lange, 1991]. To determine whether specific antibodies are produced intrathecally and not transferred passively from serum, the integrity of the blood–brain barrier must be assessed. In this study it was possible to detect blood–brain barrier dysfunction in case 1, but the high AIIgM value was consistent with intrathecal antibody production. Although the incubation period of this case was longer than reported previously for Yellow fever vaccine-associated neurotropic disease cases, 45 days, the patient showed pleocytosis, normal glucose, and an elevated protein level in CSF characteristic of a viral myelitis [Chaves et al., 2009]. At difference in case 2, who developed meningoencephalitis 4 days after vaccination, the blood–brain barrier function was normal so YFV-specific IgM antibodies detected in CSF were produced intrathecally and were not passively transferred from serum.
In this study two Argentinean cases of Yellow fever vaccine-associated neurotropic disease were confirmed; the calculated AIIgM resulted useful as a complementary tool to provide a conclusive diagnosis. The diagnosis of Yellow fever vaccine-associated neurotropic disease was done based on clinical symptoms and signs, epidemiological history, temporal proximity with the vaccination event and detection of YFV-specific IgM in CSF and in serum according with CDC case definition [WHO, 2008]. No other cause of neurological disease was identified.
Difficulty in detecting viral genome in serum and CSF samples of patients with Yellow fever vaccine-serious adverse events has been previously reported [Bae et al., 2008; Silva et al., 2010]. In a previous study, viremia was detected between 8 and 18 days after vaccination in patients with Yellow fever vaccine-severe adverse effects [Bae et al., 2008] while healthy vaccines exhibited viremia between 4 and 6 days after vaccination [Reinhardt et al., 1998]. In this study, the inability to detect viral genome could be related with the prolonged time elapsed between the moment in which samples were obtained and vaccination (case 1) or onset of illness (case 2). Suboptimal condition for incorrect storage or transportation conditions could have also affected the preservation of YFV genome.
In the present work, specific YFV antibodies were detected by specific IgM capture ELISA. This technique has been used in several studies of intrathecal immune response such as acute poliomyelitis [Roivainen et al., 1993] and Japanese Encephalitis [Chanama et al., 2005; Natividad et al., 2006].
The method applied in this study may contribute as an additional tool in the confirmation of Yellow fever vaccine-associated neurotropic disease. All the evidence for both confirmed cases indicates that YFV-specific IgM antibodies were synthesized locally within the CNS, and therefore CNS post-vaccinal YFV infection could be definitively associated to YFV vaccination.
This report points to a need for enhanced surveillance for adverse events related to YFV vaccination.




