Human platelet antigen genotype is associated with progression of fibrosis in chronic hepatitis C
Abstract
Although progression of fibrosis in the chronic hepatitis C depends on environmental, viral, and host factors, genetic polymorphisms have been associated recently with this progression, including the expression of integrins, adhesion proteins. Some integrins expressed on the platelet membrane show polymorphic antigenic determinants called human platelet antigens (HPA), where the major ones are HPA-1, -3, -5. The association between HCV infection and HPA-5b has been demonstrated. Similarly, the HPA profile could determine if HPA is related to progression of fibrosis. The goal of this study was to evaluate the association between the frequencies of HPA-1, -3, and -5 and degree of fibrosis in HCV-infected patients. Genomic DNA from 143 HCV-infected patients was used as the source for HPA genotyping by PCR-SSP or PCR-RFLP. Progression of fibrosis was evaluated using the METAVIR scoring system, and the patients were grouped according to degree of fibrosis into G1 (n = 81, with F1, portal fibrosis without septa or F2, few septa) and G2 (n = 62, with F3, numerous septa, or F4, cirrhosis). Statistical analysis was performed using the proportional odds model. The genotypic frequency of HPA-1a/1b was significantly higher in the patients in G2. To evaluate the influence of the time of infection to the development of fibrosis and its effect on the genetic factor HPA-1, 96 patients from 143 studied were evaluated considering the time of HCV infection, and these results suggest that the HPA-1a/1b genotype promotes the development of fibrosis in HCV infection with time. J. Med. Virol. 84:56–60, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Rapid progression of fibrosis in chronic hepatitis C has been related to gender, age at time of infection, alcohol abuse, coinfection with human immunodeficiency virus (HIV) and hepatitis B virus (HBV), diabetes mellitus and obesity [Poynard et al., 2001; Freeman et al., 2001; Seef, 2002].
Although the factors leading to progression of fibrosis are still unclear, studies have demonstrated the influence of host genetic factors in this process [Powell et al., 2000; Thorburn et al., 2002; Wright et al., 2003; Yee, 2004; Poujol-Robert et al., 2006] as human leukocyte antigen (HLA), polymorphisms in interleukin 10 gene (IL-10), tumor necrosis factor α (TNF-α), angiotensinogen, and transforming growth factor β1 (TGF-β1) [Powell et al., 2000]. In this way, the expression of integrins has also been associated with progression of fibrosis [Nejjari et al., 2001; Popov et al., 2008].
Integrins represent a family of heterodimeric transmembrane cellular receptors [Carloni et al., 1996] present in various cells and cellular components, including platelets [Takada et al., 2007] and hepatic stellate cells [Racine-Samson et al., 1997; Gao and Brigstock, 2004], which are already associated with HCV RNA [Hernández et al., 1998; Jiménez Sáenz et al., 2000; Hamaia et al., 2001; Almeida et al., 2004; Pugliese et al., 2004].
Some integrins of the platelet membrane express several polymorphic antigenic determinants on their surface, the human platelet antigens, HPA [Bussel and Kaplan, 1998]. Polymorphism in most of these antigens is due to the substitution of a single amino acid in the protein due to a single nucleotide substitution in the gene that encodes the membrane protein [Metcalfe et al., 2003]. Among the well-established HPA systems are HPA-1, -2, -3, -4, and -5 [Kunicki and Newman, 1992], which all except HPA-2 reside in integrins [Metcalfe et al., 2003].
Verdichio-Moraes et al. [2009] demonstrated an increased allelic frequency of HPA-5b in HCV-infected individuals when compared to a control group, suggesting a possible association between this allele and viral infection. Thus, the association between HPA antigens and fibrosis rate can determine if HPA is related to progression of fibrosis and if there is an HPA profile more frequently associated with this progression.
The goal of this study was to determine the association between the HPA-1, -3, and -5 systems [the HPA-4b allele is rare in Brazil; Toralles-Pereira et al., 2005] and the rate of liver fibrosis in antiviral therapy naive patients.
PATIENTS AND METHODS
Aliquots of EDTA-anticoagulated peripheral venous blood were collected from 143 HCV-infected patients seen at the Department of Internal Medicine, Gastroenterology Division, Botucatu School of Medicine, Sao Paulo State University, UNESP, Botucatu, SP, Brazil. Inclusion criteria were the presence of HCV infection (using RT-PCR), liver biopsy performed before the start of antiviral treatment and signed informed consent. Exclusion criteria were HBV- or HIV-positive serology, antiviral treatment before liver biopsy, and the presence of other hepatic diseases. The study was approved by the Research Ethics Committee of Botucatu School of Medicine, UNESP (statement 164/2006).
The patients were divided into two groups according to degree of fibrosis determined in liver biopsies, which were performed using the Menghini or Tru-Cut needle. The fragments were analyzed when at least eight portal spaces were present. The tissue was submitted to hematoxylin–eosin, Masson trichrome, and reticulin staining. The biopsies were analyzed by a pathologist, using the METAVIR scoring system [Poynard et al., 1997]. The groups were Group 1 (G1): patients with lower stage of fibrosis (F1, portal fibrosis without septa or F2, few septa) and Group 2 (G2): patients with higher degree of fibrosis (F3, numerous septa or F4, cirrhosis).
The patients were also classified according to the genotype of circulating HCV, where two categories were defined: genotype 1 virus and genotype non-1 virus, which included only genotypes 2 or 3. From the databank, information was obtained regarding sex, age, time of infection, defined as the time elapsed between the presumed date of infection and the date of biopsy (information evaluated in 104 of the 143 patients), and information on alcohol abuse, defined as more than 40 g per day for females and over 80 g per day for males (information evaluated in 140 of the 143 patients studied).
Genomic DNA was isolated from whole blood using the commercial Brasilica kit (LGC Biotecnologia, Sao Paulo, Brazil). HPA-1 and -3 were genotyped by PCR sequence-specific primers according to Klüter et al. [1996]. HPA-5 was genotyped by PCR restriction fragment length polymorphism according to Kalb et al. [1994].
Plasma RNA was isolated using the QIAamp Viral RNA Mini Kit (QIAGEN, Valencia, CA) and was used as the source for HCV 5′-UTR region amplification by RT-PCR using Amplicor HCV Test, version 2.0 (Roche Diagnostic Systems, Branchburg, NJ). The RT-PCR product was genotyped with INNO-LiPA® v.1.0 (Innogenetics, Ghent, Belgium). All procedures were performed according to the manufacturer's specifications.
The Hardy–Weinberg equilibrium test was carried out to determine the distribution of gene frequencies of HPA-1, -3, and -5, according to degree of fibrosis. The chi-square test was used to investigate possible associations between HPA alleles and degree of fibrosis.
Fisher's exact test was utilized to evaluate associations between the viral genotype, alcohol abuse, genotypic frequency of the HPA-1, HPA-3, HPA-5 systems, time of infection and degree of fibrosis.
Multivariate regression logistic analysis was performed to analyze variables that might have influenced progression of fibrosis (F3 or F4) and to determine an independent association of HPA genotypes with fibrosis. A Kaplan–Meier survival analysis was performed to evaluate the influence of the HPA genotypes on the progression of fibrosis (endpoint = F3 or F4) according to the time of infection.
The level of significance for all statistical tests was set at 0.05.
RESULTS
Results were analyzed using only two groups according to fibrosis degree: G1, patients with mild fibrosis [portal fibrosis without septa (F1) or few septa (F2)] and G2, patients with severe fibrosis [portal fibrosis with numerous septa (F3) or cirrhosis (F4)].
From 143 patients studied, 103 (72%) were men, with a mean age of 44.4 years and mean time of infection of 22.7 years (parameter evaluated in 104 cases due to lack of information about the probable time of infection to 34 patients). From 143 patients included in the study, 86 (60.1%) had HCV genotype 1, 57 (39.9%) genotype non-1 (2 or 3) and, 81 (56.64%) patients were included in G1 (F1 or F2) and 62 (43.36%) in G2 (F3 or F4). The abusive alcohol was observed in 66 (47.14%) patients from 140 evaluated.
There was no deviation from Hardy–Weinberg equilibrium in all HPA systems evaluated. There were no significant differences (P > 0.05) in allele frequency distribution for HPA-1, -3, and -5 between patients in G1 (F1 or F2) and G2 (F3 or F4) (Table I).
| G1 (F1 or F2) | G2 (F3 or F4) | P-value | |
|---|---|---|---|
| Allelesa | |||
| 1a | 145 | 108 | 0.5274 |
| 1b | 17 | 16 | |
| 3a | 113 | 88 | 0.8237 |
| 3b | 49 | 36 | |
| 5a | 139 | 112 | 0.2477 |
| 5b | 23 | 12 | |
| Genotypesb | |||
| 1a/1a | 69c | 47c | 0.0317 |
| 1a/1b | 07c | 14c | |
| 1b/1b | 05c | 01c | |
| 3a/3a | 42 | 35 | 0.7262 |
| 3a/3b | 29 | 18 | |
| 3b/3b | 10 | 09 | |
| 5a/5a | 58 | 50 | 0.2430 |
| 5a/5b | 23 | 12 | |
| 5b/5b | 0 | 0 | |
- G1 (n = 81, with F1, portal fibrosis without septa or F2, portal fibrosis with few septa) and G2 (n = 62, with F3, portal fibrosis with numerous septa, or F4, cirrhosis).
- a Analysis using χ2 test.
- b Analysis using Fisher's exact test.
- c Significance difference.
The progression of fibrosis was not associated with the genotype variables of HCV (P = 0.1688), alcohol abuse (P = 0.6097), or genotypes HPA-3 (P = 0.7262) and HPA-5 (P = 0.2430), based on Fisher's exact test. On the other hand, there was a significant association with the HPA-1 genotype (P = 0.0317) (Table I).
Multivariate logistic regression showed independent association between the genotype HPA-1a/1b and severe fibrosis (F3 or F4), when compared to genotype HPA-1a/1a and HPA-1b/1b with odds ratio of 3.59 (CI = 1.143–11.318) and 19.669 (CI = 1.629–237.438), respectively, independent of the viral genotype, alcohol abuse and time of infection (Table II).
| Variable | Odds ratio (95% CI) |
|---|---|
| HPA 1a/1a vs. 1a/1b | 3.597 (1.143–11.318) |
| HPA 1b/1b vs. 1a/1b | 19.669 (1.629–237.438) |
| Viral genotype 1 vs. non-1a | 2.369 (0.962–5.834) |
| Time of infection | 0.896 (0.837–0.960) |
| Abusive alcohol consumption: yes vs. no | 0.945 (0.386–2.314) |
- Model adjusted considering severe fibrosis (F3 or F4) as response variable and HPA-1 genotype, viral genotype, time of infection, and alcohol consumption as risk factors.
- a Only genotype 2 or 3.
A Kaplan–Meier survival curve showed a shorter time to develop F3 or F4 for HCV-infected individuals with the HPA-1a/1b genotype (Fig. 1).
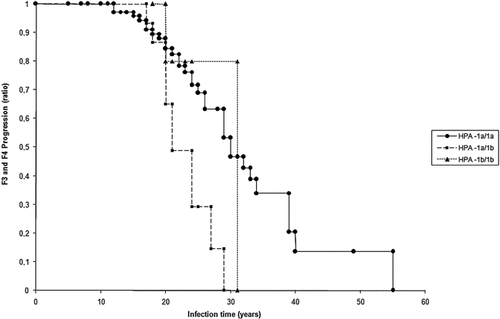
HCV-infected individuals with the HPA-1a/1b genotype showed a shorter time to develop F3 or F4 according Kaplan–Meier survival curve.
DISCUSSION
The development of fibrosis during the course of chronic infection by HCV is consequence of modifications in the hepatic microenvironment and cirrhosis represents the most advanced event of this process [Friedman, 2003; Ginès et al., 2004]. The activation of stellate cells in the liver is the principal event that leads to hepatic fibrosis, mediated by profibrogenic cytokines, including TGF-β1 [Friedman, 2000; Bissell et al., 2001], whose activation have been demonstrated are regulated by integrins [Grainger et al., 1995; Munger et al., 1999; Morris et al., 2003].
Alterations in the expression of integrins have also been associated with progression of fibrosis [Nejjari et al., 2001; Popov et al., 2008]. Studies have demonstrated the involvement of integrins in the regulation of activities of hepatic stellate cells (HSC), such as contraction, migration, proliferation, and extracellular matrix synthesis [Pinzani and Marra, 2001; Schuppan et al., 2001].
This study demonstrated the association of the HPA-1a/1b with severe fibrosis degree. This polymorphism resides in the β3 subunit of the glycoprotein of the family of integrins, expressed on the platelet surface. Conformational changes in the glycoprotein of the platelet membrane, resulting from polymorphism of the HPA-1 system could interfere with TGF-β1 activation and consequently with the more severe degree of hepatic fibrosis. In a previous study was demonstrated the association of the HPA-5b allele and HCV infection, when the patients infected with HCV and uninfected individuals were compared [Verdichio-Moraes et al., 2009], however this study do not find significance in the HPA-5 genotype and allelic frequencies between G1 and G2, probably due to all patients included in this study were HCV carriers.
These data have not described previously and, studies should continue to elucidate the true mechanisms by which HPA-1 polymorphism is involved in the progression of fibrosis and its relation to hepatic fibrogenesis, since integrins presents on the platelet membrane, are also expressed in hepatic stellate cells [Racine-Samson et al., 1997; Gao and Brigstock, 2004].




