Epstein-Barr virus, B cell lymphoproliferative disease, and SCID mice: Modeling T cell immunotherapy in vivo
Abstract
Epstein-Barr virus (EBV)-associated post-transplant lymphoproliferative disease (PTLD) arises in up to 10% of organ transplant recipients and is fatal in ∼50% of cases. PTLD can be modeled in SCID mice using EBV+ve human B lymphoblastoid cell lines (BLCLs), and the current study investigated intraperitoneal (ip) inoculation of such animals in experiments which assessed the effect of EBV-specific cytotoxic T lymphocytes (CTLs) and cytokines on PTLD growth. Ip transfer of one dose of autologous CTLs, or CD8-enriched T cells, into ip BLCL-inoculated animals significantly delayed tumor development (P = 0.001) and prevented tumor formation in a significant proportion (40%) of mice (P = 0.001). A combination of interleukin (IL)2, 7, and 15 conditioning of CTLs prior to ip injection significantly delayed ip BLCL-derived tumor formation in vivo when compared to CTLs expanded in vitro using only IL2 (P = 0.04) and prevented tumor outgrowth in a significant proportion (60%) of mice (P = 0.02). Daily ip IL2 dosing of ip CTL-inoculated mice significantly delayed tumor development in vivo (P = 0.004) and prevented tumor outgrowth in a significant proportion (78%) of mice (P = 0.02) when compared to animals dosed with vehicle only. In SCID mice, autologous CTLs, and CD8-enriched T cells, have significant capacity to hinder development of PTLD-like tumors. Whilst studies are needed to delineate the role of cytokine conditioning and CD4-enriched T cells, the results suggest that IL2 plays a key role in supporting CTL funtion in vivo. J. Med. Virol. 83:1585–1596, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Novel therapeutic regimes require validation in suitable animal models to assess efficacy and safety before clinical studies can be undertaken. The severe combined immunodeficient (SCID) mouse is a small animal model that has been used extensively to study novel treatments for human cancer. For example, these humanized animals constitute an in vivo model in which the treatment of Epstein-Barr virus (EBV)-associated post-transplant lymphoproliferative disease (PTLD) can be investigated.
EBV is a ubiquitous agent that infects persistently over 90% of adults worldwide [Henle and Henle, 1966]. It is usually acquired in childhood when infection is generally asymptomatic although if deferred until young adulthood, infectious mononucleosis may result [Crawford et al., 2006]. Following primary infection, EBV establishes a life long persistent infection in resting memory B lymphocytes [Babcock et al., 1998].
EBV immune control is mediated by CD4+ve and CD8+ve major histocompatibility complex (MHC)-restricted EBV-specific cytotoxic T lymphocytes (CTLs) against latent and lytic viral proteins [Bogedain et al., 1995; Steven et al., 1997; Bickham et al., 2001; Moss et al., 2001] with CTL responses to the EBNA3 antigens being immunodominant in healthy EBV seropositive individuals [Murray et al., 1990, 1992] where up to 5% of circulating CD8+ve CTLs are committed to EBV immune control [Hislop et al., 2002].
In organ transplant recipients, iatrogenic immunosuppression reduces EBV-specific CTL activity, and this may result in EBV-driven B cell proliferation and, ultimately, PTLD. PTLD develops in up to 10% of organ graft recipients and is characterized by rapid onset, aggressive behavior, and approximately 50% mortality despite current treatment [Williams and Crawford, 2006]. The major risk factors are the degree and duration of immunosuppression required for graft maintenance and primary EBV infection following transplantation [Thomas et al., 1995].
The majority of PTLD express all EBV latent antigens and the tumor is generally susceptible to EBV-specific CTL destruction. Adoptive CTL immunotherapy provides rapid, targeted treatment that is well tolerated and effective in preventing and treating PTLD [Rooney et al., 1998; Haque et al., 2007]. However, a better understanding of the optimum CTL regime is required to realize its full potential. To this end, our laboratory has used the SCID mouse to model PTLD [Johannessen et al., 2000].
Due to an autosomal mutation, SCID mice are unable to produce functional B and T cell clones [Bosma et al., 1983] and, therefore, the animals cannot reject human xenografts. Following transfer of human EBV+ve B cells, mice develop human PTLD-like tumors (hu-SCID) that reflect patient lesions including expression of all EBV latent antigens [Mosier et al., 1988; Johannessen et al., 2002]. Various hu-SCID models have been established, and it is not entirely clear which is most suitable for translational treatment studies. Peripheral blood mononuclear cells (PBMCs) from EBV-seropositive healthy donors give rise to PTLD-like lesions in a proportion of cases when cells are transferred intraperitoneally (ip) into SCID mice [Picchio et al., 1992; Johannessen et al., 2000]. However, inter-donor variability exists, and PBMCs from 25% of blood donors do not form tumors in this hu-PBMC-SCID model. In contrast, in vitro EBV-infected B lymphoblastoid cell lines (BLCLs) give rise consistently to PTLD-like lesions in the animals [Cannon et al., 1990; Rowe et al., 1991] and such hu-BLCL-SCID models are suited to answer questions of treatment efficacy. To this end, BLCLs can be inoculated ip.
This study used the ip hu-BLCL-SCID model to assess the efficacy of CTLs in vivo and the role of a panel of cytokines in supporting CTL function. The ip hu-BLCL-SCID prevention model was a practical small (SCID mouse) animal model in which to study CTL function. Using this model, CD8+ve T cell subsets prevent PTLD outgrowth and interleukin (IL)2 alone, or in combination with IL7 and 15, enhances this effect.
MATERIALS AND METHODS
Blood Donations
Buffy coats were obtained with ethical approval from the Scottish National Blood Transfusion Service (SNBTS), Edinburgh (UK). EBV serostatus was determined by anti-VCA IgG antibody titer using a standard indirect immunofluorescence assay [Henle and Henle, 1966]. Donor PBMC MHC tissue typing was carried out at SNBTS (Prof. M Turner) or the Anthony Nolan Research Institute (London; Prof. A Dodi) using PCR-based methods.
Establishment of BLCLs
Concentrated EBV was added to PBMCs in a total volume of 1 ml of culture medium [CM: RPMI 1640 supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 10% v/v fetal calf serum (FCS)], incubated at 37°C for 1 hr, washed (in Hank's buffered saline solution), resuspended (106 cells/ml; with ciclosporin), and plated out in a 24-well plate. During culture in vitro, cultures were fed CM every 2–3 days. At 4 weeks, cells were assessed directly under a light microscope for signs of immortalization, immunophenotyped, and % viability determined using trypan blue. A viability of ≥70% was considered acceptable.
T Cell Subset Selection
T cell subsets were positively selected from CTLs using EasySep® kits (StemCell Technologies, Grenoble, France) following the manufacturer's instructions. Briefly, 100 µl/ml EasySep® selection mix was added to 1 × 108 cells/ml in EasySep® buffer, incubated for 15 min followed by addition of 50 µl/ml EasySep® magnetic dextran iron nanoparticles, a further incubation for 10 min, and increase of final sample volume to 2.5 ml (EasySep® buffer). Following 5 min on a magnet, supernatant was removed, 2.5 ml of buffer added to the selected cells followed by further magnetic separations (repeated thrice). The final cell fraction was resuspended in CM. The median purity of the CD4+ve and CD8+ve T cell subsets obtained was 53% and 88%, respectively.
Generation of EBV-Specific CTLs
EBV-specific CTLs were established as described previously [Wilkie et al., 2004]. Briefly, a 40:1 PBMC:γ-irradiated (4,000 rads) BLCL mixture was cultured at 1 × 106/ml in 20% v/v FCS CM. Ten days later, T cells were restimulated at a ratio of 4:1 T cells:γ-irradiated autologous BLCL followed 4 days later by addition of 20 IU/ml of recombinant IL 2 (rIL2). From this timepoint (14 days) onwards, T cell cultures were restimulated weekly with γ-irradiated autologous (4:1 ratio) BLCLs together with a thrice weekly dose of rIL2. At time of weekly stimulation, T cell concentration was adjusted to 1 × 106 cells/ml to ensure optimum growth conditions.
Flow Cytometry
Flow cytometry was carried out using standard methods. Briefly, working dilutions of directly conjugated monoclonal antibodies (mabs) were added to cells suspended in 50 µl of flow buffer (FB: phosphate-buffered saline containing 1% w/v bovine serum albumin, 5 mM EDTA, and 0.1% w/v sodium azide) and the mixture incubated at 4°C for 20 min in the dark. Following FB washes, immunostained cells were resuspended in 1× CellFIX™ (BD Biosciences, Oxford, UK). Flow cytometric acquisition (and analysis of 1,000–10,000 acquired events/sample) was performed using the “CellQuest Software” on a FACSCalibur machine (Becton Dickinson, Oxford, UK).
Cytotoxicity Assay
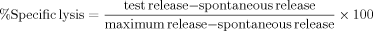
Histopathology and Immunohistochemistry
Paraffin wax-embedded tissues were dewaxed and rehydrated. Hematoxylin and eosin (H&E) staining was carried out using standard methods. Immunostaining included an initial antigen retrieval step (using “Target Retrieval Solution” or “Antigen Retrieval Solution”; DakoCytomation, Ely, UK). Sections were immunostained with primary mabs (in “Antibody Diluent, Background Reducing”; DakoCytomation) for 30 min at room temperature, washed in tris-buffered saline (TBS), and “EnVision™” (DakoCytomation) and “NovoLink™ Polymer Detection System” (Novocastra, Newcastle Upon Tyne, UK) kits used to visualize bound antibody following manufacturers' instructions. Sections were counterstained in Mayer's Hemalum, dehydrated (if appropriate), mounted, and coverslipped. For quantification, numbers of mab-labeled and -unlabeled cells were counted in five high power (×1,000 under oil) fields and results expressed as % mab+ve cells.
EBER In Situ Hybridization (ISH)
“Epstein-Barr Virus (EBER) PNA Probe/Fluorescein” and “PNA ISH Detection Kit” (DakoCytomation) kits were used in accordance with the manufacturer's instructions. Briefly, 0.1 mg/ml proteinase-K was applied to dewaxed and rehydrated paraffin wax-embedded sections for 30 min. A FITC-labeled PNA probe complimentary to EBER1 and 2 was applied at 55°C for 1.5 hr, sections immersed in a 55°C working solution (1:60 in pure water) of “Stringent Wash” for 25 min, and incubated with “Anti-FITC/AP” for 30 min. Bound probe was visualized by an AP “Substrate” (BCIP/NBT) followed by counterstaining in Mayer's Hemalum, mounting, and coverslipping.
SCID Mice
CB.17 scid/scid mice were bred at the University of Edinburgh under specific pathogen free conditions in individually ventilated microisolator cages and handled in microbiological class 2 safety cabinets. All animal work was carried out under relevant Home Office Project and Personal Licences in accordance with the Home Office “Animals (Scientific Procedures) Act 1986,” and monitored by Home Office Inspectors and Named University Veterinary Surgeons. Mice were observed twice daily and culled if sick (defined as disinterest in their surroundings, hunched back, respiratory distress, and/or ruffled fur), or after 100 days. Tissues were harvested at necropsy and fixed in neutral buffered formalin (NBF).
Statistical Analysis
Advice on statistical analysis and presentation of data was sought from public health departments of the London School of Hygiene & Tropical Medicine and the University of Edinburgh. Tumor incidence was analyzed by the Fisher's exact test (two-tailed P-value). Time to tumor was assessed by the Kruskall, Mann–Whitney U-test, and Spearman's tests. “GraphPad Prism” software (GraphPad Software Inc, La Jolla) was used for data analysis and P ≤ 0.05 was considered significant. Datapoints on survival curves in Figures 2 and 4 reflect donor-derived results obtained as triplicates since groups of three SCID mice were used for each test and control group. Standard Error (SE; shown as error bars on graphs) was calculated as: SE = SDn−1/√n (where SD is the standard deviation and n denotes the number of samples analyzed). Similar to survival curves, data presented in Tables II and III represent results obtained for each donor in triplicates (i.e., each control and test group consisted of three SCID mice).
RESULTS
The aim was to assess the ip hu-BLCL-SCID model for tumor prevention as a tool to assess safety and efficacy of novel CTL-based immunotherapy. The model was used to investigate the role of T cell subsets and cytokines in PTLD therapeutic regimes.
A panel of 25 BLCLs (and T cell subsets; see below) was generated by in vitro EBV infection of PBMCs from 25 healthy EBV-seropositive blood donors (denoted “donor 1–25”; for MHC typing results, please refer to Table I). BLCLs were transferred ip into SCID mice to study tumor prevention.
| Donor No. | A | B | C | DR | ||||
|---|---|---|---|---|---|---|---|---|
| Allele 1 | Allele 2 | Allele 1 | Allele 2 | Allele 1 | Allele 2 | Allele 1 | Allele 2 | |
| 1 | 03 | 31 | 27 | 45 | 01 | 06 | NT | NT |
| 2 | 24 | 68 | 14 | 15 | 03 | 08 | NT | NT |
| 3 | 23 | 23 | 44 | 49 | 04 | 07 | NT | NT |
| 4 | 02 | 29 | 44 | 44 | NT | NT | 01 | 07 |
| 5 | 02 | 32 | 07 | 14 | NT | NT | 07 | 15 |
| 6 | 01 | 31 | 08 | 44 | NT | NT | 07 | 17 |
| 7 | 01 | 03 | 07 | 14 | 07 | 08 | NT | NT |
| 8 | 01 | 02 | 35 | 73 | 04 | 15 | NT | NT |
| 9 | 03 | 68 | 40 | 44 | 03 | 08 | NT | NT |
| 10 | 01 | 29 | 07 | 08 | 07 | 07 | NT | NT |
| 11 | 01 | 03 | 07 | 07 | NT | NT | 04 | 15 |
| 12 | 02 | 68 | 50 | 60 | NT | NT | 04 | 17 |
| 13 | 02 | 11 | 27 | 44 | NT | NT | 01 | 14 |
| 14 | 03 | 03 | 51 | 60 | NT | NT | 04 | 15 |
| 15 | 11 | 32 | 27 | 62 | NT | NT | 01 | 11 |
| 16 | 02 | 32 | 44 | 44 | 05 | 16 | 04 | 07 |
| 17 | 02 | 02 | 08 | 44 | 05 | 07 | 03 | 13 |
| 18 | 01 | 02 | 08 | 44 | 05 | 07 | 04 | 15 |
| 19 | 02 | 31 | 35 | 51 | NT | NT | 07 | 17 |
| 20 | 02 | 03 | 40 | 44 | 03 | 05 | NT | NT |
| 21 | 23 | 25 | 44 | 45 | NT | NT | 04 | 12 |
| 22 | 01 | 32 | 08 | 35 | NT | NT | 01 | 17 |
| 23 | 02 | 02 | 37 | 44 | NT | NT | 01 | 07 |
| 24 | 01 | 02 | 07 | 57 | NT | NT | 07 | 09 |
| 25 | 01 | 02 | 08 | 57 | NT | NT | 01 | 07 |
- BLCL, B lymphoblastoid cell line; MHC, major histocompatibility complex; No, number; NT, not tested.
Establishment of ip Hu-BLCL-SCID Model: Tumor Prevention
In this model, tumor prevention is used as experimental read-out. Firstly, the minimum cell number required for consistent BLCL-driven ip tumor formation in vivo was assessed by inoculating groups of three animals ip with 1 × 106, 2 × 106, or 4 × 106 BLCL cells from our BLCL panel. All mice inoculated ip with either 2 × 106 or 4 × 106 BLCL cells developed macroscopic ip tumors in a median time of 6.5 and 6 weeks, respectively, whereas, an inoculum of 1 × 106 BLCL cells produced ip tumors in 40% of animals in a median time of 6 weeks. Therefore, in this model, ip tumors were induced in all further experiments with a standard 2 × 106 BLCL dose. In parallel control experiments, PBMCs from three EBV-seronegative healthy donors did not give rise to ip tumors. Previously, the effect of depleting endogenous (murine) NK cells (using rabbit anti-mouse-ASGM1 antiserum) on ip tumor development in SCID mice has been investigated and found not to impact significantly on tumor formation in vivo [Johannessen et al., 2000]. Therefore, we do not administer routinely an anti-NK antiserum to our animals.
Analysis Of Tissues and Tumors
Paraffin wax-embedded tissue sections from lung, liver, spleen, and tumor tissue from a panel of 10 ip mice inoculated with BLCL from different donors (donors 1–10; see Table I) were screened using H&E staining, anti-human CD45 and CD20 immunostaining, in situ hybridization for EBERs, and immunostaining for EBV latent (EBNA2 and LMP1) and immediate early (IE) lytic (BZLF1) antigens (see Fig. 1). Macroscopic ip tumors formed primarily as solitary nodules at the undersurface of the liver. (1) H&E: Sections were scored for distortion of normal tissue morphology and cellular infiltrations. Large immunoblastic tumor cells were detected consistently in ip tumor tissue only. (2) Human CD45 and CD20: Scattered human CD45+ve, CD20+ve cells were found only rarely in lung, liver, and spleen. Conversely, such cells formed ip tumor tissue. (3) EBERs: Scattered EBER+ve cells were found only rarely in lung, liver, and spleen. Conversely, such cells formed ip tumor tissue. (4) EBV antigens: EBER+ve cells in tumor tissue were found to express the latent EBNA2 and LMP1 antigens with the occasional cell expressing the IE lytic BZLF1 lytic antigen.
Immunophenotyping Of SCID mouse tumors: Human leukocyte markers and EBV transcripts (paraffin wax-embedded sections). A,B: Photographs of the peritoneal cavity of ip inoculated control (A: suspension medium only) and test (B: BLCL) SCID mice taken at necropsy. Arrow indicates ip BLCL-derived tumor in test mouse (B). C (×100), D (×400): Photomicrographs of H&E staining of tumor sections showing large lymphoblastoid tumor cells with large nuclei (blue hematoxylin staining) and scant cytoplasm. E,F (×400): Photomicrographs of immunostaining of the human pan-leukocyte marker CD45 on tumor sections using an alkaline phosphatase (AP) label. E: CD45+ve human cells (red membrane staining) counterstained with Mayer's hemalum. F: The conjugate control with counterstained cells only. G,H (×400): Photomicrographs of immunostaining of the human pan-B cell marker CD20 on tumor sections using a peroxidase (HRP) label. G: CD20+ve human cells (brown membrane staining) counterstained with Mayer's hemalum. H: The conjugate control with counterstained cells only. I,J (×400): Photomicrographs of in situ hybridization for EBERs on tumor sections using an AP label. I: EBER+ve cells (dark blue/black nuclear staining) on a probed tumor section which was counterstained with Mayer's hemalum (blue nuclear staining). J: The counterstained unprobed (negative) control tumor section. The panels show a single mouse experiment but are representative of several experiments.
Characterization Of In Vitro Expanded T Lymphocytes
EBV-specific T cells were expanded from PBMCs from a panel of 25 EBV seropositive donors (donors 1–25; see Table I) by culture in vitro. Cells were harvested after 7–17 weeks (median: 10 weeks) of culture at which time the percentage of cells expressing the following markers were delineated by flow cytometry and the medians calculated: CD4+ve median 6%, CD8+ve median 84%, CD56+ve median 1%, and CD19+ve <1%. In parallel, cytotoxic function was assessed by a 4 hr standard 51Cr-release assay with target cells including autologous and allogeneic (MHC mismatched) BLCLs and K562. Median specific cell lysis at a 10:1 effector:target ratio were 36% for the autologous BLCL, 6% for the allogeneic BLCL, and 7% for K562. The differences between median % specific lysis for autologous versus allogeneic targets and the NK cell target K562 were significant (Mann–Whitney U-test: P < 0.0001 on both occasions).
Model For Tumor Prevention
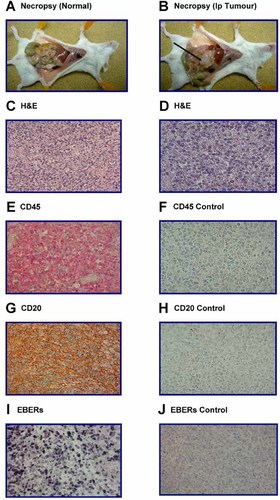
Using 10 donors (donors 1–10; see Table I), 2 × 106 BLCL were inoculated ip into each mouse in groups of three SCID mice followed 1 hr later by inoculation ip of either suspension medium (vehicle) or a median of 50 × 106 (range: 40 × 106–50 × 106) autologous CTLs. Animals were culled when showing signs of sickness (see Materials and Methods Section), or at a pre-determined time limit of 100 days [in line with previous experience; Johannessen et al., 2000]. On each occasion, a sick mouse was found to harbor ip macroscopic tumor at necropsy. The percentages of mice in each group that developed ip tumors are shown in Table II. Individual results (together with Log-rank test results) are shown in Figure 2. Tumors formed in 29 (97%) and 26 (87%) out of 30 mice injected with vehicle only and CTLs, respectively, and, thus, there was no significant difference between the two groups of animals in terms of the proportion of mice that developed ip tumors (Fisher's exact test: P = 0.353). In contrast, mice inoculated with CTLs survived significantly longer than control animals (Mann–Whitney U-test: P = 0.001; Log-rank test: P = 0.003) and, therefore, their tumor development was delayed when compared to control mice. However, this effect was lost when ip CTL inoculation was deferred for 3 weeks following ip BLCL transfer (Log-rank test: P = 0.60; Data not shown). Furthermore, administration of mismatched CTLs did not have a significant impact on tumor development in vivo (Log-rank test: P = 0.77; Data not shown) indicating that tumor prevention is MHC restricted.
| Inoculum | Medium only | CTL | CD4+ | CD8+ |
|---|---|---|---|---|
| Number of tumors/number of mice injected (%) | 29/30 (97) | 26/30 (87) | 25/30 (83) | 18/30* (60) |
- CTL, cytotoxic T lymphocytes; ip, intraperitoneal. Data reflects results obtained for each donor in triplicates (i.e., groups of three SCID mice).
- * Statistically significant difference (P = 0.001) when compared with mice inoculated with medium only.
% survival using CTLs and T cell subsets (datapoints reflect results in triplicates; Standard error, SE, is shown on each column as a vertical bar).
Role of CD8+ve T Cell Subsets
Using the same 10 donor CTLs (donors 1–10; see above), we enriched for CD8+ve T cells using magnetic beads. For each donor, 2 × 106 BLCL were inoculated ip into each mouse in groups of theee SCID mice followed 1 hr later by inoculation ip of CD8+ve enriched T cell populations. Thus, each of three mice received ip a median of 37 × 106 (range: 3 × 106–50 × 106) CD8+ve enriched T cells. Animals were culled when showing signs of sickness (see Materials and Methods Section), or at a pre-determined time limit of 100 days. On each occasion, a sick mouse was found to harbor ip macroscopic tumor at necropsy. The percentages of mice in each group that developed ip tumors are shown in Table II. Individual results (together with Log-rank test results) are shown in Figure 2A–C. Tumors developed in 18 out of 30 (60%) mice injected with CD8-enriched T cells. Comparing the results with control mice inoculated with vehicle only (see Table II and Fig. 2), significantly fewer animals injected with CD8-enriched T cells developed ip tumors (Fisher's exact test: 0.001). Furthermore, CD8-inoculated mice survived significantly longer than controls (Log-rank test: P = 0.0002). Whilst groups of mice were also inoculated with a median of 7 × 106 (range: 3 × 106–67 × 106) autologous CD4-enriched T cells, it was not possible to compare the efficacy of CD4-inoculated mice with that of CD8-injected ones since CD4-enrichment yielded fewer cells in vitro than the CD8 one. Thus, although no significant difference was found between mice inoculated with CD4-enriched T cells and control animals injected with vehicle only when assessing tumor formation (Fisher's exact test: P = 0.195) and survival (Log-rank test: P = 0.101), a valid comparison with CD8-injected mice could not be carried out.
Taken together, mice inoculated with CTLs, or CD8-enriched T cells, survived significantly longer than control animals (Mann–Whitney U-test: P = 0.001 for both analysis; Log-rank test: P = 0.003 and 0.0002, respectively). Conversely, the use of CD4-enriched T cells did not impact significantly on tumor development in vivo (Log-rank test: P = 0.101). Since the proportion of CD4+ve cells in the transferred inoculum differed between donors, we re-analyzed the results using data obtained when using CD4-enriched cells from the five donors whose CD4-enriched populations contained the highest level of CD4+ve cells (a median of 74%; range: 70–97%; see Fig. 2D). The transfer of CD4-enriched cells from those individuals did not impact significantly on tumor development in vivo (Log-rank test: P = 0.35) which is in line with the overall results described above.
The in vitro cell seperation manipulation per se did not affect significantly CTL performance in vivo as determined in parallel control studies. For each of three donors, 2 × 106 BLCLs were inoculated ip into each mouse in two groups of three SCID mice each. Whilst 50 × 106 unfractionated autologous CTLs were inoculated ip into each animal in the control group, each mouse in a test group received 50 × 106 CTLs that had been fractionated into CD4+ve and CD8+ve T cell subpopulations using magnetic beads and recombined in the original CTL subset proportions. Unfractionated CTLs and fractionated/recombined CTLs had a similar effect in vivo (Log-rank test: P = 0.71; Data not shown).
In the present study, the CD4- and CD8-enriched T cell populations were not pure populations so calculations of the total number of these cells inoculated in each experiment were carried out. A noncorrelation was found between the numbers of CD4+ve or CD8+ve, T cells (included in the inoculated CTL, CD4-enriched, or CD8-enriched T cells), and the average % survival (in days) beyond controls (Spearman's test: P > 0.05 for all analyzes). Similar results were obtained when analyzing the CD8:CD4 ratio of cells inoculated (Spearman's test: P > 0.05 for all analyzes). Therefore, it does not appear that survival of SCID mice treated with autologous CTLs or T cell subsets, is a direct function of the number of transferred CD4+ve or CD8+ve T cells or their ratio. The level of CTL cytotoxicity against the autologous BLCL (as assayed in vitro by 51Cr-release studies) did not correlate with survival of CTL-inoculated SCID mice (Spearman's test: P = 0.17; Data not shown) and was, therefore, not a useful parameter for predicting survival.
In Vivo Trafficking of Human T Cells
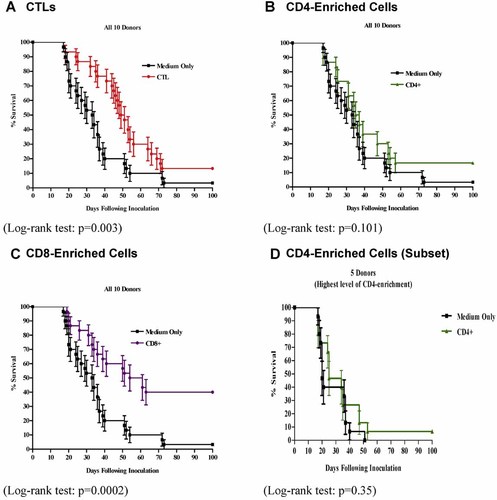
We analyzed samples of lung, liver, spleen, and tumor harvested from the CTL-treated hu-BLCL-SCID mice (see above) to look for human T cell trafficking within these tissues. The percentage of specific antibody+ve cells on immunostained sections was assessed by attributing a score of (−), (+), 1+, 2+, and 3+, indicating that none, <1%, 1–29%, 30–60%, or >60% of all cells counted were antibody+ve cells, respectively. Tissues were obtained at necropsy from 32 ip (harvested at 17–73 [median: 32] days after ip CTL inoculation) hu-BLCL-SCID mice (see Fig. 3). Human CD3+ve T cells were found in 17 out of 32 (53%) tumor samples tested. Whilst 2 of these 17 (12%) samples contained CD4+ve T cells, 11 (65%) had detectable CD8+ve T cells, and 1 (6%) contained CD4+ve and CD8+ve T cells. Three (18%) samples contained only CD3+ve cells. Overall, T cells comprised <1–29% of each T marker-positive tumor section. Human cells were not detected in control mice. The data suggest that human T cells are able to home to human tumor tissue in vivo. Since human CD45+ve cells were detected only very rarely in lung, liver, and spleen tissue, these samples were not analyzed further. Immunostaining of the murine pan-nucleated cell marker MHC1 on tumor sections showed no (or the very occasional) murine cell underlining further that the lesions were human in origin (Data not shown).
Immunophenotyping Of SCID mouse tumor tissue: Human leukocyte and cytotoxic molecule markers. A,B (×400): Photomicrographs of immunostaining of the human panleukocyte marker CD45 on tumor sections using an alkaline phosphatase (AP) label. A: CD45-positive human cells (red membrane staining) counterstained with Mayer's hemalum. B: The conjugate control with counterstained cells only. C,D (×400): Photomicrographs of immunostaining of the human pan-T cell CD3 marker on tumor sections using an AP label. C: CD3-positive human cells (red membrane staining) counterstained with Mayer's hemalum. D: The conjugate control with counterstained cells only. E,F (×400): Photomicrographs of immunostaining of the human T helper cell CD4 marker on tumor sections using an AP label. E: CD4-positive human cells (red membrane staining) counterstained with Mayer's hemalum. F: The conjugate control with counterstained cells only. G,H (×400): Photomicrographs of immunostaining of the human T cytotoxic CD8 marker on tumor sections using an AP label. G: CD8-positive human cells (red membrane staining) counterstained with Mayer's hemalum. H: The conjugate control with counterstained cells only. I,J (×400): Photomicrographs of immunostaining of the cytolytic granule molecule perforin on tumor sections using a peroxidase (HRP) label. I: Perforin-positive human cells (brown cytoplasmic staining) counterstained with Mayer's hemalum. J: The conjugate control with counterstained cells only. Arrows point to examples of specific antibody +ve cells.
Serial sections from the 17 ip tumors shown to contain CD3+ve T cells (see above) were stained for perforin and granzyme B cytoloytic molecules (see Fig. 3). Six out of 17 (35%) tumors contained granzyme B+ve cells and a further 8 out 17 (47%) contained perforin+ve cells. Overall, 14 out of 17 (82%) tumor samples expressed one or other cytoxic granule molecule with no dual expression noted.
Cytokine Conditioning
Cytokines that signal through the common γ-chain (IL2, 4, 7, 15, and 21) are important for the homeostasis of CD4+ve and CD8+ve T cells with the ability to enhance their homing and promote their expansion, function, and survival in vivo [Ku et al., 2000; Schluns et al., 2000]. The effect on survival of CTL cytokine conditioning in vitro prior to transfer in vivo into ip hu-BLCL-SCID mice was examined. CTL lines (day −1) from 5 donors (donors 11–15; see Table I) were cultured in medium containing 20% v/v FCS and human IL2 (20 IU/mL) with the addition of IL7, 15, 21, 7 and 15, or 7 and 21, at a final concentration of 10 ng/mL for 24 hr prior to washes, reconstitution in suspension medium, and inoculation into mice [day 0; Parada et al., 1998; Ayyoub et al., 2002].
Two million cells from each donor BLCL were inoculated ip into each mouse in groups of three SCID mice each. This was followed 1 hr later by inoculation ip of suspension medium (vehicle) only into one group of three animals, and 50 × 106 autologous CTLs that had been conditioned with cytokines for 24 hr in vitro into further groups of three SCID mice. Individual results showing the percentage survival over time (in days) together with log-rank test results is shown in Figure 4A–E. When SCID mice were inoculated with CTLs conditioned in vitro with IL2 together with IL7, 15, 21, 7 and 15, or 7 and 21, animals that received CTLs conditioned with a combination of IL7 and 15 survived significantly longer than control mice inoculated with CTLs conditioned with IL2 only (Log-rank test: P = 0.04). Furthermore, tumors developed in 6 out of 15 (40%) mice that received such IL7/15-conditioned CTLs which is significant protection against tumor development when compared with tumor formation in 13 out of 15 (87%) control mice inoculated with IL2-conditioned CTLs (Fisher's exact test: P = 0.02; see Fig. 4D). In contrast, there was no significant difference between the survival curves of animals that received CTLs conditioned in vitro with IL2 together with IL7, 15, 21, or 7 and 21, when compared with control mice (Log-rank test: P = 0.96, 0.67, 0.52, and 0.75, respectively).
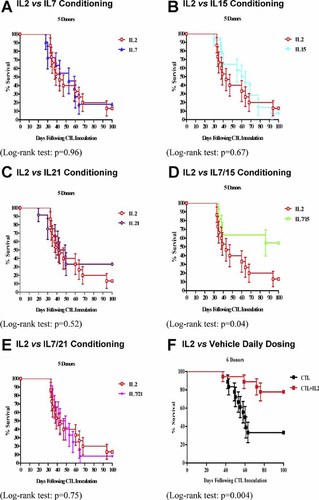
% survival using cytokine conditioned CTLs (datapoints reflect results in triplicates; Standard error, SE, is shown on each column as a vertical bar).
The EBV-specific cytotoxicity in vitro of CTL lines on day 0 was assessed and no significant difference found between median % specific lysis when comparing autologous targets conditioned with IL2 alone, or IL2 combined with IL7, 15, 21, 7 and 15, or 7 and 21 (Kruskal–Wallis test: P = 0.74; Data not shown).
In order to assess further the role of IL2 for CTL function, 2 × 106 BLCL cells from one of six donors (donors 11–16; see Table I) were inoculated ip into each mouse in groups of three SCID mice each. One hour later, 50 × 106 autologous CTLs that had been conditioned for 24 hr in vitro with IL2 were inoculated ip into groups of three SCID mice. This was followed by inoculations ip of either 250 IU of IL2 or vehicle only. Mice were dosed daily for 14 days following ip transfer of BLCL cells. Individual results showing the percentage survival over time (in days) together with log-rank test results is shown in Figure 4F and the percentages of mice in each group that developed ip tumors are shown in Table III. CTL-inoculated animals that received IL2 daily survived significantly longer than control mice dosed with vehicle only (Log-rank test: P = 0.004). Tumors developed in 4 out of 18 (22%) such mice dosed with IL2, whereas, 12 out of 18 (67%) control mice dosed with vehicle only developed ip tumors which is a significant difference (Fisher's exact test: P = 0.02). Taken together, these results suggest a key role for IL2 in supporting CTL function in vivo.
| Inoculum | Medium only | CTL | CTL+IL2 |
|---|---|---|---|
| Number of tumors/number of mice injected (%) | 12/18 (67) | 12/18 (67) | 4/18* (22) |
- CTL, cytotoxic T lymphocytes; IL, interleukin; ip, intraperitoneal. Data reflects results obtained for each donor in triplicates (i.e., groups of three SCID mice).
- * Statistically significant difference (P = 0.02) when compared with mice inoculated with medium or CTL only.
DISCUSSION
The importance of animal modeling has not diminished over the years despite efforts to offer alternative in vitro options, and pre-clinical data obtained in suitable animal models are still vital to guide translation into the clinical setting. Whilst SCID mice are a small animal model that is widely used to study human cancer (including PTLD), there is as yet no International Standard defining how the model should be used to ensure high quality data. In the current study, we used an ip hu-BLCL-SCID mouse tumor prevention model because of its suitability for testing of novel immunotherapy against PTLD.
Boyle et al. [1993] engrafted SCID mice ip with BLCL and showed that autologous CTL administered simultaneously (or 7 days later) delayed significantly tumor formation, whereas, mismatched CTLs failed to do so, indicating the MHC restriction of CTLs. In the present study, administration of autologous, but not allogeneic, CTLs in the ip hu-BLCL-SCID model similarly delayed tumor development and improved survival time, and further support for this tumor prevention model comes from studies by DiMaio et al. [1995] and Buchsbaum et al. [1996].
The current study demonstrates that ip hu-BLCL-SCID mice give rise reliably to macroscopic EBV+ve human B immunoblastic ip tumors portraying full (unrestricted) latent virus gene expression with the occasional tumor cell in lytic cycle. In this prevention model, ip tumor formation is the experimental read-out and autologous CTLs can be detected in tumor tissue. In parallel studies, we have also investigated the subcutaneous (sc) hu-BLCL-SCID model in which transfer of 2 × 106 BLCL sc into flanks of SCID mice gives rise consistently to visible sc PTLD-like tumors that can be measured directly. Theoretically, such a model can be used to assess the efficacy of novel PTLD immunotherapy when treatment is delivered by intravenous (iv) inoculation. However, such a tumor regression model poses technical challenges that undermine its use. In particular, in the sc model, sc tumors may arise at different timepoints after inoculation making the experimental protocol for T cell therapy difficult to plan. Furthermore, the transfer of large T cell inocula iv can be problematic and it is difficult to ensure successful injection of the large T cell numbers required for analysis. The ip hu-BLCL-SCID model used, herein, circumvents these difficulties and is technically straight-forward to manage. However, since ip tumor development cannot be monitored clinically in this model, it is a prevention model with BLCLs and CTLs given 1 hr apart, a situation that does not reflect accurately the clinical setting. Whilst we have introduced MRI imaging of our animals to mimic better the clinical (patient) situation with a view to provide a noninvasive, non-destructive method of monitoring ip tumor development in longitudinal studies on the same animals, this approach has yet to prove its value in face of technical challenges in demarcation of small lymphoid lesions by MRI scanning of SCID mice. Taken together, translational data can be obtained in the ip prevention SCID mouse model that is difficult to generate in other SCID mouse models and such data can be used to inform clinical (patient) protocols.
Using the ip prevention hu-BLCL-SCID model, CD8+ve T cell subpopulations from CTLs from across the donor panel delayed significantly tumor development, and tumor formation was entirely prevented in 40% of mice (see Table II). Boyle et al. [1993] also used CD8-enriched CTL populations in this model with significant effect on tumor development. Whilst inoculation of CD4-enriched T cells did not have a significant impact on survival in this model (see Fig. 2), the data is too limited to draw conclusions from this set of experiments. Interestingly, in the hu-PBMC-NOD/SCID model (NOD/SCID animals are deficient in NK as well as B and T cells), treatment of mice with a depleting anti-human CD4 mab results in loss of both CD4+ve and CD8+ve T cells [Wagar et al., 2000] suggesting at least a supporting role to CD8+ve T cells for CD4+ve lymphocytes. Since the CD4-enriched T cell populations transferred into our animals consisted of smaller total T cell numbers than the CD8-enriched inocula, and there was no significant difference in the rate of ip tumor development between mice inoculated with bulk CTLs and animals that received CD4-enriched cells only, the latter may still have an anti-tumor effect or work to prevent CD8+ve T cell-mediated anti-tumor mechanisms (see Results Section). Thus, the data must be interpreted with a certain degree of caution.
CD4+ve T cells show cytotoxic function in vitro and in vivo [Appay, 2004], and since PTLD cells express MHC2 molecules [Thomas et al., 1990], direct CD4+ve T cell-mediated cytotoxicity is possible. However, CD4+ve T cells also have a variety of helper functions and in a recent clinical trial using allogeneic “best MHC match” CTL treatment for PTLD, a significant trend for a better outcome with higher numbers of CD4+ve T cells in infused CTL lines was interpreted as the effect of the few CD4+ve T cells in the CTLs in enhancing the survival in vivo of the CD8+ve population [Haque et al., 2007].
Discrepancies exist between the murine and human situation. Furthermore, in the ip hu-BLCL-SCID model, the most effective combination of CD4+ve and CD8+ve T cells has not yet been identified. Interestingly, studies in our laboratory have shown that PBMC-inoculated mice (hu-PBMC-SCID) deplete of T regulatory (Treg) cells develop PTLD-like tumors at a similar rate to Treg-replete PBMC populations which conflicts with current ideas that Treg cells play a major role in tumor immune escape (Data not shown). These results highlight further the discrepancies that exist between the murine and human situation.
Human T cells were detected in T cell treated animals for up to 64 days which is in line with observations by others underlining that T cells can home to PTLD-like tumors in SCID mice. Thus, Lacerda et al. [1996] showed preferential homing of CD8+ve T cells to autologous (and not MHC mismatched) tumor tissue as did imaging studies by Koehne et al. [2003] using radioactive labeling of human EBV-specific T cells. Whilst our immunostaining results support a role for the cytotoxic molecules perforin and granzyme B, the data must be interpreted with a certain degree of caution.
A combination of IL7 and 15 enhanced the ability of CTLs from across the donor panel to mediate tumor prevention in vivo although individually IL7, 15, and 21 did not (Fig. 4). Furthermore, a combination of IL7 and 21 did not have an impact in vivo. It is postulated that a combination of IL7 and 15 improved the ability of the in vitro conditioned T cells to home to, and mediate destruction of, human PTLD-like tumor cells in vivo which is in line with recent in vivo data using IL15 [Klebanoff et al., 2004]. However, since a correlation between the effects of the cytokines with expression of their relevant receptors by CTLs at the time of in vitro cytokine conditioning was not carried out, the results should be interpreted with a degree of caution. Whilst in vitro cytokine conditioning did not affect significantly CTL cytotoxicity in vitro, additional results from this study [as well as data from clinical studies in our laboratory; Haque et al., 2007] suggest that in vitro cytotoxicity does not correlate with CTL function in vivo.
Previous studies have given conflicting results when examining a possible role of cytokine administration in sustaining transferred T cells in vivo [Boyle et al., 1993; Baiocchi and Caligiuri, 1994; Rencher et al., 1994; Baiocchi et al., 2001]. However, in line with data from other laboratories [Baiocchi and Caligiuri, 1994; Baiocchi et al., 2001], the current study underlines a supporting role for IL2 in facilitating CTL survival and function in vivo (see Fig. 4F and Table III) although the results do not exclude possible contribution from IL2-mediated activation of murine NK cells. Whilst the results suggest inclusion of IL2 in immunotherapeutic regimes using CTLs against PTLD, a degree of caution must be exercised in the use of the cyotkine in a patient population due to its ability to cause side effects.
Our laboratory has successfully infused PTLD patients with EBV-specific CTLs on a “best MHC match” basis [Haque et al., 2002, 2007]. Although this proof-of-principle trial showed that CTL therapy could succeed, a mechanism for bypassing MHC restriction would facilitate its dissemination to the clinic. A chimeric T cell receptor (cTCR) can be constructed that directs T cells against a novel surface antigen in a nonMHC restricted manner [Schumacher, 2002; Mansoor et al., 2005], and currently our laboratory employs such an approach to re-target CTLs to EBV-associated cancers. This novel therapeutic approach will be tested in the pre-clinical ip hu-BLCL-SCID model presented in the current study.
Acknowledgements
We are indebted to the staff of the Biomedical Research Resources (The University of Edinburgh) for maintaining the SCID mice used in this project. We also gratefully acknowledge the technical assistance of Dr. K.A. McAulay, Mr. A. Timpson, and Ms. N. Blair.




