Genotype diversity of group A rotavirus strains in children with acute diarrhea in urban Burkina Faso, 2008–2010
Abstract
In this study, the diversity of G and P genotypes of rotavirus strains in Burkinabe children were examined. Between November 2008 and February 2010, 447 stool samples were collected from children <5 years of age with acute diarrhea visiting hospital in Ouagadougou. Group A rotavirus was previously detected in 151/447 (33.8%) of the samples tested by an immunochromatographic test and these samples were now tested further for rotavirus G and P genotypes by RT-PCR. Of these, the rotavirus type genes were amplified by RT-PCR for 140/151 (92.7%) samples and G and P genotypes were successfully determined for 81 (57.9%) and 130 (92.9%) samples, respectively. The most prevalent G genotypes were G1, 34/140 (24.3%), and G9, 21/140 (15%), while the predominant P genotypes were P[6], 56/140 (40%), and P[8], 54/140 (38.6%). Among the single infections, 63/140 (45%), the predominant G/P combinations were: G1P[8] (33%), G9P[8] (29%), and G2P[6] (14%). The unusual strains G1P[9] (3%), G12P[6] (3%), G10P[6] (2%), and G2P[8] (2%) were also detected. In a high number of strains 61/140 (43.6%), the G genotype could not be determined and mixed infections were determined in 17/140 (12.1%) of strains identified. This study highlights the high diversity and presence of unusual rotavirus strains in children in Burkina Faso. J. Med. Virol. 83:1485–1490, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Rotavirus is a major cause of acute gastroenteritis in infants and young children worldwide [Desselberger et al., 2006]. The World Health Organization (WHO) estimates that globally 527,000 deaths occur each year among children as a result of rotavirus infection [WHO, 2007]. Although the incidence of infection among children in developed and developing countries is similar, outcomes vary widely with about 82% of fatalities occurring in less developed regions [Parashar et al., 2003, 2006]. Furthermore, rotavirus infections are an important cause of hospitalization in young children, causing considerable social and economic burden in low-income countries [Hoshino and Kapikian, 2000; Parashar et al., 2003].
Group A human rotaviruses are non-enveloped 70 nm RNA viruses belonging to the Reoviridae family and exhibit considerable genetic diversity. Rotaviruses have a three capsid layer structure [Estes, 2001] with the outer capsid made up of a protease sensitive protein designated as VP4 and a glycoprotein designated as VP7. The nature of two outer capsid proteins of the virus, VP4 and VP7, elicits the production of neutralizing antibodies to the virus [Hoshino et al., 1985; Offit et al., 1986] and allows to define the P (for P sensitive) and G (for glycoprotein) serotypes of the virus. Currently, 23 G genotypes and 32 P genotypes have been described, based on nucleotide sequence variation [Matthijnssens et al., 2008a,b, 2009; Ursu et al., 2009; Collins et al., 2010]. Global epidemiologic surveys have identified G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8] as the most common global G/P genotype combinations associated with diarrhea in humans [Glass et al., 2006; Estes and Kapikian, 2007]. However, recent studies in developing countries have shown increased identification of a high proportion of rotavirus strains with unusual G/P combinations, which may have implications for vaccine efficacy [Gentsch et al., 2005; Santos and Hoshino, 2005; Todd et al., 2010]. Several studies conducted in Ghana, Ivory Coast and Nigeria, close neighbors of Burkina Faso, have reported the high incidence of unusual G/P genotype combinations with the emergence of G9 strains as important cause of gastroenteritis [Armah et al., 2003, 2010a; Akran et al., 2010; Aminu et al., 2010]. Currently, two rotavirus vaccines [Rotarix (GSK Biologicals, Rixensart, Belgium) and RotaTeq (Merck & Co. Inc., West Point, PA)] have been licensed and implemented in immunization programs for children in many countries in Americas and Europe [Ruiz-Palacios et al., 2006; Vesikari et al., 2006]. Results from recently efficacy trials of these vaccines in Africa—Ghana, Mali, Kenya [Armah et al., 2010b], and in South Africa and Malawi [Madhi et al., 2010]—have shown good efficacy of these vaccines and protection in infants. Results from these studies have culminated in the recommendation by the WHO for the introduction of rotavirus vaccines for all infants and inclusion of these vaccines in the extended program of immunization (EPI) of countries [WHO, 2009]. The WHO further recommends the putting in place surveillance programs to help in the monitoring of rotavirus strains and the determination of vaccine effectiveness.
Although a number of studies have been published on rotavirus genotypes circulating in West Africa, little is known about the situation in Burkina Faso. To date, only one report on rotavirus genotypes isolated 10 years ago in Burkina Faso has been published [Steele et al., 2010], which reported the predominance of the unusual rotavirus strain G2P[6].
The present work is a continuation of a previous study on rotavirus epidemiology in Burkina Faso, in which group A rotavirus prevalence was found to be 33.8% [Bonkoungou et al., 2010]. The objective of this study was to determine the diversity of G and P genotypes of the rotavirus strains circulating among children with acute diarrhea in Burkina Faso during two consecutive rotavirus seasons from 2008 to 2010.
MATERIALS AND METHODS
Rotavirus Positive Samples
Rotavirus positive samples used for the present study were those in which group A rotavirus was detected in a previous study, for which full details of the sampling and testing procedures as well as clinical and sociodemographic information of the children are published [Bonkoungou et al., 2010]. Briefly, 447 children under the age of 5 years, who had diarrhea and/or were admitted to the Centre Médical avec Antenne Chirugicale (CMA) du Secteur 30 of Ouagadougou between November 2008 and February 2010 were enrolled in the study. Of the 447 stool samples tested by immunochromatographic (SD Bioline Rota/Adeno®; Standard Diagnostics, Inc., Kyonggi-Do, South Korea), 150 (33.8%) revealed the presence of group A rotavirus. These rotavirus-positive samples were submitted for G and P genotyping conducted at the WHO Regional Rotavirus laboratory (RRL) at the Department of Electron Microscopy and Histopathology, Noguchi Memorial Institute for Medical Research of the University of Ghana.
RNA Extraction and Reverse Transcription
Rotaviral RNA was extracted from 500 µl of 10% faecal suspensions in PBS and purified with the RNaid Kit (BIO 101, La Jolla, CA) [Gentsch et al., 1992]. The extracted RNA was used for a semi-nested multiplex RT-PCR after specific priming with VP7 and VP4 consensus primer pairs [Gouvea et al., 1990; Gentsch et al., 1992]. Briefly, 1 µl of the specific primer pair (Beg9/End9) for VP7 or (Con3/Con2) for VP4 was added to 8 µl of the extracted RNA, heated at 94°C for 5 min to denature the RNA and immediately chilled on ice for 2 min. This was followed by the addition of 3.2 µl of the reverse transcriptionase (RT) reaction mix (62.5 µM of each dNTP, Promega; and 5 U of AMV reverse transcriptase; Promega Corporation, Madison, WI). The RT was carried out by incubation at 42°C for 30 min. This was followed by a 30 cycles of PCR (1 min denaturing at 94°C, 2 min annealing at 42°C, and 3 min extending at 72°C) followed by a final extension cycle at 72°C for 7 min. The reaction mixture contained 1.5 U Taq polymerase (Gibco BRL, Life Technologies, Gaitherburg, MD).
G and P Typing PCR
G typing was performed by using a seminested PCR method as described by Gouvea et al. [1990] and adapted by Iturriza-Gomara et al. [2004] and Banerjee et al. [2007]. Briefly, in the first round, the full-length VP7 gene was obtained with primer pair Beg9/End9. This was followed by a second-round multiplex PCR, which incorporated primer End9, the G-type-specific primer RVG9, and primers aBT1, aCT2, aET3, aDT4, aAT8, aFT9, G10, and G12, which are specific for G types 1, 2, 3, 4, 8, 9, 10, and 12, respectively. For P typing, a similar method using a seminested PCR adapted from the method described by Gentsch et al. [1992] was used. In the first-round PCR, the full-length VP8* gene products were obtained using the consensus primers Con2 and Con3. This was then followed by a second-round typing PCR which incorporated primer Con3 and primers 1T-1, 2T-1, 3T-1, 4T-1, and 5T-1, which are specific for types P[8], P[4], P[6], P[9], and P[10], respectively. All PCR products were examined by gel electrophoresis in 1.2% agarose gels containing 4 µg of ethidium bromide/ml and the G and P types were determined by the molecular weights of the amplicons.
RESULTS
Genotyping Results
Of the 151 group A rotavirus positive samples, 140 (92.7%) had sufficient sample material for further characterization by RT-PCR. Of these, seven (5%) could not be assigned either a G and P type specificity (non-typeable samples). The G genotype was successfully determined for 79 (56.4%) and P genotype for 130 (92.9%) rotavirus samples (Table I).
| P type | G type | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G9 | G10 | G12 | Mixeda | NTG | All | |
| P4 | 0 | 1 (0.7) | 0 | 0 | 0 | 0 | 0 | 0 | 4 (2.9) | 5 (3.6) |
| P6 | 3 (2.1) | 9 (6.4) | 0 | 1 (0.7) | 2 (1.4) | 1 (0.7) | 2 (1.4) | 1 (0.7) | 37 (26.4) | 56 (40) |
| P8 | 21 (15) | 1 (0.7) | 0 | 2 (1.4) | 18 (12.9) | 0 | 0 | 3 (2.1) | 9 (6.4) | 54 (38.6) |
| P9 | 2 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (1.4) |
| Mixedb | 5 (3.6) | 1 (0.7) | 1 (0.7) | 0 | 1 (0.7) | 0 | 0 | 1 (0.7)c | 4 (2.6) | 13 (9.3) |
| NTP | 3 (2.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 (5.0) | 10 (7.1) |
| All | 34 (24.3) | 12 (8.6) | 1 (0.7) | 3 (2.1) | 21 (15) | 1 (0.7) | 2 (1.4) | 5 (3.6) | 61 (43.6) | 140 (100) |
- NTG, non-typeable for G; NTP, non-typeable for P.
- Data are number (%) of strains (n = 140).
- a The mixed G type found in this study were (0.7%) for each, G1G9[P8], G1G10[P4], G1G10[P8], and G1G10[P6].
- b The mixed P type found in this study were G1[P6]P[8](3.6%), P[6]P[8](1.4%) and (0.7%) for each [P8]P[10], P[4][P6]P[8], P[4][P8]P[9], G1[P4]P[8], G1P[4][P8]P[9], G1P[4][P6]P[8], G2P[6][P8]P[9], and G3[P6]P[8].
- c G-mixed–P-mixed type found in this study was G1G2 P[6][P8](0.7%).
Frequencies of G and P Genotypes
Considering G and P types separately in the 140 positive samples characterized by RT-PCR, the most common G types determined were G1 (24.3%), G9 (15%), and G2 (8.6%). Other genotypes determined were G4 (2.1%), G3 (0.7%) and the newly emerging genotypes G12 (1.4%), G10 (0.7%). Similarly, the commonest P types detected were P[6] (40%) and P[8] (38.6%). Other P types detected were P[4] (3.6%) and the unusual VP4 type P[9] (1.4%) (Table I).
Distribution of G/P Combinations
Both G and P type specificities could be assigned successfully to 63 (45%) of the samples characterized (Fig. 1). The common infecting rotavirus strains detected during the period of study in children with acute diarrhea in Burkina Faso were G1P[8] (33%), G9P[8] (28%), and G2P[6] (14%). Less common types detected were: G1P[6] (5%), G4P[8] and G9P[6] (3% each), G4P[6] and G2P[4] (2% each). In addition, in this study, unusual rotavirus strains bearing the genotypes G1P[9], G12P[6], G10P[6], and G2P[8] were detected as shown in Figure 1. Thirteen of the strains characterized had mixed P genotypes and five were of mixed G genotypes. Only one sample exhibited both mixed G and P genotypes (Table I). In 54 (38.6%) samples, the P genotype could be determined but the G type could not be determined. Likewise in three (2.1%) samples, the G type could be determined but the P type was not and in seven (5%) both the G and P types could not be determined.
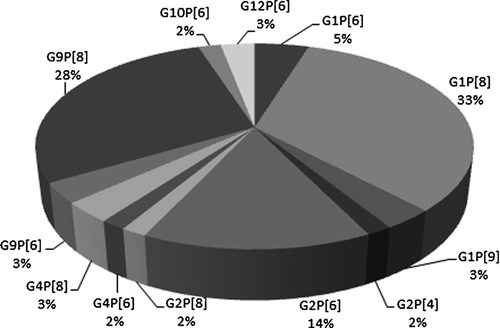
Frequencies (%) of rotavirus G/P combinations among 63 samples for which both G and P were determined between November 2008 and February 2010.
Seasonal Distribution of Rotavirus Genotypes
During the study period, rotavirus G/P genotype combinations (single types) were detected during December to March, corresponding to the dry season and relatively cool and cold nights. The most common prevailing strain changed over the 2-year study period. During the first rotavirus season, the most common rotavirus genotypes detected were G1P[8] and G2P[6] whilst during the second season the most common genotype was G9P[8], (Fig. 2).
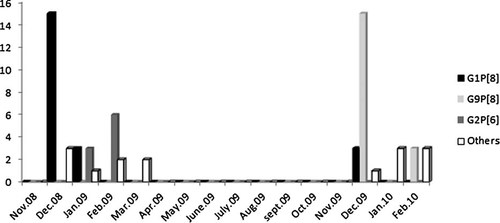
Monthly distribution of rotavirus G/P combinations (single types) circulating in Ouagadougou, Burkina Faso, November 2008–February 2010. Others: G1P[6], G1[P9], G2[P4], G2[P8], G4[P6], G4P[8], G9[P6],G10[P6], G12[P6].
DISCUSSION
This study reports on the detection and characterization of rotavirus strains in Ouagadougou during 2008–2010, representing the longest-term study for rotavirus surveillance in Burkina Faso. The present work is a continuation of a previous study on rotavirus epidemiology in Burkina Faso [Bonkoungou et al., 2010], which revealed that rotavirus accounted for 33.8% of diarrheal episodes. Genotyping of human rotavirus strains revealed a great diversity of G and P genotypes circulating in urban Burkina Faso. Previous rotavirus studies indicated that G1P [8], G2P [4], G3P [8], G4P [8], and G9P [8] represent 74% of globally identified strains [Gentsch et al., 2005]. However, these strains are less prevalent comprising of only 37% of those strains in Africa [Todd et al., 2010]. In the current survey, these five most common genotypes globally constituted 64% of detected rotavirus strains in Ouagadougou. G1 strains were the most commonly detected (24.3%) in the 140 positive samples, frequently in combination with P[8]. The second G specificity found most frequently in this study was G9 (15%), which emerged as the fifth most important human genotype. G9 was detected mostly in combination with P[8], as has been reported elsewhere in the West African sub-region [Armah et al., 2003, 2010c].
The unusual G/P type G2P[6] was found to be the third most common genotype 14% (Fig. 1) associated with diarrhea in children in Burkina Faso. Adah et al. [2001] and Salu et al. [2003] reported about a decade ago the existence of this genotypes in the region. Recently, the importance of G2P[6] in Burkina Faso has been reported [Steele et al., 2010]. Also studies in Ghana and Cote d'Ivoire, which are close neigbors to Burkina Faso, reported high prevalence of G2P[6] and the researchers suspected it to be an emerging strain in the sub-region of West Africa [Armah et al., 2001, 2010a; Akran et al., 2010]. Other unusual G/P combinations recently reported in Africa [Steele et al., 2003; Page et al., 2010] were found in low frequencies, notable combinations being G12P[6], G1P[6], and G2P[8] which represented two (3%), one (2%), and one (2%), respectively, of the single G/P combination infections (Fig. 1). These genotypes were probably secondary to reassortment among co-circulating human genotypes [Iturriza-Gómara et al., 2001; Maunula and Von Bonsdorff, 2002] and were the most common unusual strains found in West Africa also previously [Armah et al., 2010c]. The presence of G9 strains and unusual isolates in Burkina Faso and its neighboring countries may be due to the continuous population movement between these countries.
Mixed infections have already been reported representing about 12.4% of isolated rotavirus strains in Africa [Todd et al., 2010], as was also the case in the present study (12.1%). This study also documents the detection of rotavirus strains normally associated with animals (cattle and a cat); two G1P[9], one G10P[6], and three G10 mixed infections. G10 and P[9] have been recovered sporadically from humans in various geographical areas. In developing countries, the lack of adequate sanitation and potable water supplies and the close association of humans with domestic and farm animals could give rise to reassortment between human and animal strains with the potential of cross-species infections [Cook et al., 2004].
During this study, 43.6% of samples were G untypable despite several attempts. This might be due to common strains with accumulated point mutations not recognized by the primers used or new unidentified G types, and only sequence analysis of VP7 PCR products will confirm this.
Rotavirus occurs mostly during the period from December to March, corresponding to the dry season and relatively cool and cold nights, as has been reported in Burkina Faso and elsewhere in West Africa [Binka et al., 2003; Rodrigues et al., 2007; Akran et al., 2010; Bonkoungou et al., 2010]. During the study period, rotavirus strains varied from one season to another: the most common strains G2P[6] and G9P[8] were identified during the first rotavirus season and G1P[8] during both rotavirus seasons (Fig. 2). Several studies have shown that large fluctuations in the genotype distribution of human rotaviruses occur continuously from 1 year to another or from one place to another [Tcheremenskaia et al., 2007; Yang et al., 2008].
In conclusion, this study, in addition to describing the rotavirus G and P genotypes circulating in human infections in Burkina Faso, highlights the existence of significant diversity of rotavirus strains with unusual G and P combinations. These data will be useful for making an informed decision about the introduction of rotavirus vaccine in Burkina Faso and provides baseline data for future vaccine studies.
Acknowledgements
We thank the staff of the Electron Microscopy and Histopathology Department of NMIMR for their technical assistance.




