Human papillomavirus infection among women with cytological abnormalities in Switzerland investigated by an automated linear array genotyping test
Abstract
Limited data are available describing human papillomavirus (HPV) genotype distribution among females with cytological abnormalities in Switzerland. Cervical cell specimens obtained from 5,318 women were screened routinely by liquid-based Pap smear. All specimens with cellular abnormalities were analyzed subsequently for HPV DNA by the Linear Array HPV genotyping test. Cellular abnormalities were found in 202 (3.8%) specimens, of which 150 (74.3%) were positive for high-risk (HR) HPV. HR-HPV was detected in 20 (60.6%; 95% CI, 43.7–75.4%) of 33 specimens with atypical squamous cells of undetermined significance compared to 98 (72.1%; 95% CI, 64–78.9%) of 136 low-grade squamous intraepithelial lesions and 32 (97%; 95% CI, 83.4–99.9%) of 33 high-grade squamous intraepithelial lesions. The cumulative prevalence of HR-HPV other than HPV 16 and 18 was significantly higher than HPV 16 and/or 18 lesions with atypical squamous cells and low-grade lesions and was comparable in high-grade squamous intraepithelial lesions. The most common HR-HPV genotypes were HPV 16 (15.2%), HPV 31 (12.1%), HPV 58 (12.1%), HPV 51 (9.1%), and HPV 59 (9.1%) in women with atypical squamous cells, HPV 16 (25%), HPV 51 (16.9%), HPV 52 (11.8%), HPV 31 (9.6%), and HPV 56 (8.1%) in women with low-grade lesions (LSIL) and HPV 16 (57.6%), HPV 18 (18.2%), HPV 31 (15.2%), HPV 52 (12.1%), and HPV 58 (6.1%) in women with high-grade lesions (HSIL). J. Med. Virol. 83:1370–1376, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Cervical cancer is still the second most common cancer in women worldwide [Schiffman et al., 2007]. In the communities where cervical cytology screening programs have been introduced, marked reductions in cervical cancer incidence have followed [Herrero, 1996]. In Switzerland, a yearly Pap smear based routine cervical cancer screening has been available since 1960s to all women aged 20 years or older and is covered by statutory health insurance. National recommendations include an annual cervical cytology screening and, after three consecutive negative results, extending the interval to every 2–3 years. Each person in Switzerland has an obligatory health insurance and every woman has an equal chance of being tested. The incidence of cervical cancer in Switzerland is low, ranging between 5.7 and 9.3 per 100,000 women [Petignat et al., 2005].
It is known that almost all cases of cervical cancer are caused by persistent infection with about 15 genotypes of high-risk (HR)-human papillomavirus (HPV) [Muñoz et al., 2003; De Sanjosé et al., 2007; Schiffman et al., 2007]. The most important of these genotypes are HPV 16 and HPV 18, which account for nearly 70% of all invasive cervical cancers with minor variations in the percentage between continents [Smith et al., 2007].
In order to reduce the prevalence of cervical cancer, HR-HPV screening is already used or proposed in some countries adjunctively with cervical cytology to screen women aged 30 years and older to assess the presence or absence of high-risk HPV genotypes [Castle et al., 2009; Meijer et al., 2009]. Recently, the Linear Array (LA) HPV genotyping test (Roche Diagnostics, Rotkreuz, Switzerland) was optimized and automated, making it suitable for routine HPV genotyping and molecular epidemiology [Dobec et al., 2009].
As of January 2008, two HPV vaccines have been approved for use in many countries and national and regional immunization programs aimed at young adolescent girls have been implemented widely [Paavonen et al., 2009]. So far, HPV vaccination programs have been implemented in all Cantons of Switzerland and about one-third of the 11- to 19-year-old females have been vaccinated [Bundesamt für Gesundheit, 2010].
In spite of the progress achieved in the prevention of cervical cancer worldwide, limited population-based data are available describing HPV genotype distribution and its association with cellular abnormalities in Switzerland [Petignat et al., 2005; Dobec et al., 2009]. Such studies are needed to predict how HPV vaccination and HPV-based screening will influence cervical cancer prevalence [Wheeler et al., 2009].
The purpose of the present study was to analyze the HPV genotype distribution among females with cellular abnormalities in Switzerland undergoing routine cervical screening by liquid-based Pap smear, using the LA HPV genotyping test.
MATERIALS AND METHODS
Study Population and Specimen Collection
The study cohort comprised consecutive cervical specimens of 5,318 women living in the German speaking part of Switzerland. Of 3,955,200 females living in Switzerland, 2,514,933 are situated in the studied area.
All samples were collected from private gynecology practices between May and June 2007 and sent for routine cytological examination by liquid-based Pap smear.
Specimens were collected using a Cervex brush (Rovers Medical Devices B.V., Oss, The Netherlands), rinsed into ThinPrep vials containing PreservCyt fixative solution (ThinPrep® liquid Pap test system, Hologic Corp. [previously Cytyc Corp.], Marlborough, MA) and submitted to cytological screening prior to HPV genotyping.
Liquid-based cytology slides were read initially by the ThinPrep Imager (ThinPrep Imaging System, Hologic Corp.). This computerized system for reading slides, is a relatively new technology applied to liquid-based cytology. The Imager identifies 22 fields of interest most likely to contain abnormal cells, which are then examined by a cytotechnologist. In this study the 22 fields of interest were examined manually by two cytotechnologists, using an automated microscope to locate possible abnormalities. Those slides considered normal were reported as such. If a slide was unsatisfactory or abnormal it was rescreened by a pathologist. The pathologist's diagnosis provided the report for satisfactory slides in which one or both cytologists found an abnormality.
For quality assurance purposes a 10% random selection of smears reported as negative or unsatisfactory were rescreened according to European guidelines for quality assurance in cervical cancer screening [Arbyn et al., 2010].
A positive cytological test was defined as a test that indicated the presence of atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LSIL) or high-grade squamous intraepithelial lesions (HSIL). Cytological diagnoses were classified according to the Bethesda nomenclature system and were interpreted without prior knowledge of the women's HPV status [Jones, 1995; Solomon et al., 2001].
HPV detection and genotyping were performed subsequently by the Linear Array (LA) HPV test (Roche Diagnostics) in all specimens with cytological abnormalities.
Linear Array HPV Genotyping Test (LA-HPV)
The linear array HPV genotyping Test (Roche Diagnostics) is a qualitative in vitro test for the detection of HPV in clinical specimens.
The test amplifies target HPV DNA by the polymerase chain reaction (PCR) followed by nucleic acid hybridization and allows the detection of 37 HPV DNA genotypes (Table I).
| Carcinogenic risk | HPV genotype |
|---|---|
| High risk | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 (MM9), 82 (MM4) |
| Probably high risk | 26, 53, 66 |
| Low risk | 6, 11, 40, 42, 54, 61, 70, 72, 81, CP6108 |
| Indeterminate risk | 55, 62, 64, 67, 69, 71, 83 (MM7), 84 (MM8), IS39 |
DNA Extraction
For automated DNA extraction from cervical cells in PreservCyt Solution, a procedure has been developed specifically for the Cobas AmpliPrep instrument (Roche Diagnostics) as described elsewhere [Dobec et al., 2009].
PCR Amplification of HPV DNA
The Linear Array HPV Genotyping Test (Roche Diagnostics) amplifies a 450 base pairs fragment within the polymorphic L1 region of the HPV genome using a mixture of primers allowing amplification of DNA from 37 different HPV genotypes (Table I). In addition, a fragment of the cellular β-globin gene was amplified simultaneously in order to assess cellular adequacy as well as the extraction and amplification steps [Dobec et al., 2009].
A positive (HPV genotype 16) and a negative control, provided in the kit, were included in each run.
Hybridization and Detection
Following PCR amplification, the denatured HPV and β-globin amplicons were hybridized to a genotyping strip coated with HPV and β-globin probe lines using the Tecan ProfiBlot 48 instrument (Tecan, Männedorf, Switzerland) as described previously [Dobec et al., 2009].
Result Interpretation
The strips were scanned and analyzed using the Linear Array Image Analysis Software LAIAS v.2.3.1 (Roche Diagnostics). HPV data interpreted with the LAIAS software were exported electronically from the LAIAS platform to the laboratory information system [Dobec et al., 2009].
Statistical Analysis
For the evaluation of the test results a 95% confidence interval (CI) for proportions based on Agresti and Coull was applied [Agresti and Coull, 1998].
RESULTS
Cytological Results
Out of 5,318 liquid-based slides, 18 (0.3%) were unsatisfactory. Cellular abnormalities were found in 202 (3.8%) of the 5,300 remaining cervical specimens. Of these abnormalities, 33 (0.6%) were interpreted as ASCUS, 136 (2.6%) as low-grade squamous intraepithelial lesions, and 33 (0.6%) as high-grade squamous intraepithelial lesions. No invasive cervical cancer cases were detected in this study. The age range of women with cellular abnormalities was from 17 to 77 years (mean age, 35 years; standard deviation, 12 years; median, 33 years). All samples with cellular abnormalities were tested subsequently for HPV DNA.
Epidemiological Data
HPV DNA prevalence according to cytological result
As shown in Table II, any high-risk (HR) and low-risk (LR) HPV DNA was detected in 186 (92.1%) and HR-HPV in 150 (74.3%) of 202 women with cellular abnormalities.
| Cytological result | No. of specimens tested (%) | Any HPV positive | HR-HPV positive | ||
|---|---|---|---|---|---|
| N | Prevalence, % (95% confidence interval) | N | Prevalence, % (95% confidence interval) | ||
| ASCUS | 33 (16.33) | 25 | 75.8 (58.8–87.4) | 20 | 60.6 (43.7–75.4) |
| LSIL | 136 (67.33) | 129 | 94.9 (89.6–97.7) | 98 | 72.1 (64–78.9) |
| HSIL | 33 (16.33) | 32 | 97 (83.4- > 99.9) | 32 | 97 (83.4- > 99.9) |
| Total | 202 (100) | 186 | 92.1 (87.4–95.2) | 150 | 74.3 (67.8–81.4) |
- ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
The prevalence of HR-HPV DNA was 60.6% (95% CI, 43.7–75.4%) in specimens of women with ASCUS, 72.1% (95% CI, 64–78.9%) in low-grade squamous intraepithelial lesions and 97% (95% CI, 83.4–99.9%) in high-grade squamous intraepithelial lesions.
HR-HPV genotypes were significantly more frequent in high-grade lesions compared to low-grade lesions and specimens with atypical squamous cells.
Distribution of individual HPV genotypes according to cytological results
Table III shows the prevalence of 15 HR-HPV (as listed in Table I) and LR-HPV vaccine genotypes 6 and 11 in 202 samples of women with cytological abnormalities.
| HPV genotype | ASCUS (n = 33), N (%) | LSIL (n = 136), N (%) | HSIL (n = 33), N (%) | Total (n = 202), N (%) |
|---|---|---|---|---|
| 6a | 1 (3) | 5 (3.7) | 0 (0) | 6 (3) |
| 11a | 1 (3) | 2 (1.5) | 0 (0) | 3 (1.5) |
| 16 | 5 (15.2) | 34 (25) | 19 (57.6) | 58 (28.7) |
| 18 | 1 (3) | 8 (5.9) | 6 (18.2) | 15 (7.4) |
| 31 | 4 (12.1) | 13 (9.6) | 5 (15.2) | 22 (10.9) |
| 33 | 2 (6.1) | 4 (2.9) | 1 (3) | 7 (3.5) |
| 35 | 1 (3) | 3 (2.2) | 0 (0) | 4 (2) |
| 39 | 0 (0) | 8 (5.9) | 0 (0) | 8 (4) |
| 45 | 2 (6.1) | 4 (2.9) | 1 (3) | 7 (3.5) |
| 51 | 3 (9.1) | 23 (16.9) | 1 (3) | 27 (13.4) |
| 52b | 2 (6.1) | 16 (11.8) | 4 (12.1) | 22 (10.9) |
| 56 | 2 (6.1) | 11 (8.1) | 2 (6.1) | 15 (7.4) |
| 58 | 4 (12.1) | 9 (6.6) | 2 (6.1) | 15 (7.4) |
| 59 | 3 (9.1) | 7 (5.1) | 2 (6.1) | 12 (5.9) |
| 68 | 1 (3) | 1 (0.7) | 1 (3) | 3 (1.5) |
| 73 | 2 (6.1) | 9 (6.6) | 0 (0) | 11 (5.4) |
| 82 | 0 (0) | 1 (0.7) | 0 (0) | 1 (0.5) |
- ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
- For clinical specimens that tested positive for HPV 33, HPV 35, and/or HPV 58, co-infection with HPV genotype 52 could not be ruled out.
- a Low-risk HPV genotypes.
- b Specimens interpreted as HPV 52 positive hybridized with a cross-reactive 52/33/35/58 probe but tested negative for HPV 33, HPV 35, and/or HPV 58 DNA.
The most common HR-HPV genotypes were HPV 16 (15.2%), HPV 31 (12.1%), HPV 58 (12.1%), HPV 51 (9.1%), and HPV 59 (9.1%) in women with ASCUS, HPV 16 (25%), HPV 51 (16.9%), HPV 52 (11.8%), HPV 31 (9.6%) and HPV 56 (8.1%) in women with low-grade squamous intraepithelial lesions and HPV 16 (57.6%), HPV 18 (18.2%), HPV 31 (15.2%), HPV 52 (12.1%), and HPV 58 (6.1%) in women with high-grade squamous intraepithelial lesions.
Cumulative prevalence of HPV 16, HPV 18, and other non-HPV 16/18 HR-HPV genotypes according to cervical cytology
Cumulative prevalence of HPV 16 and/or 18 (one or both genotypes) compared to non-HPV 16/18 HR-HPV genotypes (one or more of the 13 non-HPV 16/18 HR-HPV genotypes) is presented in Figure 1. Non-HPV 16/18 HR-HPV genotypes were detected more frequently (51.5%; 95% CI, 35.2–67.5%) than HPV 16 and/or 18 (18.2%; 95% CI, 8.2–34.8%) in specimens with ASCUS. In low-grade squamous intraepithelial lesions, non-HPV 16/18 HR-HPV genotypes were found in 61.8% (95% CI, 53.4–69.5%) and HPV 16 and/or 18 in 29.4% (95% CI, 22.4–37.6%). In high-grade squamous intraepithelial lesions no significant difference was observed in the prevalence of non-HPV 16/18 HR-HPV (45.5%; 95% CI, 29.8–62%) compared to HPV 16 and/or 18 (69.7%; 95% CI, 28–41%).
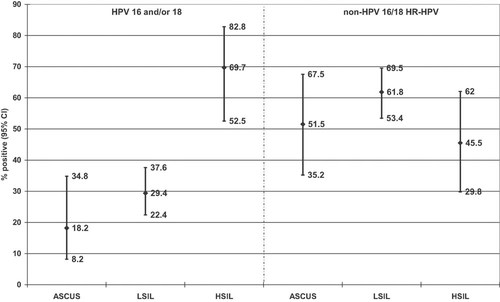
Cumulative prevalence of human papillomavirus (HPV) 16 and/or 18 in comparison to non-HPV16/18 high-risk (HR) HPV genotypes in women with atypical squamous cells of undetermined significance (ASCUS, n = 33), low-grade squamous intraepithelial lesions (LSIL, n = 136), and high-grade squamous intraepithelial lesions (HSIL, n = 33). The results are shown as proportions of positive testing with their 95% confidence intervals [modified Wald, based on Agresti and Coull, 1998].
HR-HPV single and multiple infections
One HR-HPV genotype only was detected in 79 (52.7%) and infection with multiple HPV genotypes was found in 71 (47.3%) of 150 HR-HPV positive specimens.
Single HR-HPV genotypes showed an increasing prevalence and were found in 8 (40%; 95% CI, 21.8–61.4%) of 20 HR-HPV positive patients with ASCUS, in 50 (51%; 95% CI, 41.3–60.7%) of 98 HR-HPV positive patients with low-grade squamous intraepithelial lesions and in 21 (65.6%; 95% CI, 48.2–79.7) of 32 HR-HPV positive patients with high-grade squamous intraepithelial lesions.
The most frequent single HR-HPV genotype was HPV 16, detected in 27 (18%), followed by HPV 31 in 14 (9.3%), HPV 52 in 10 (6.7%), HPV 51 in 7 (4.7%), and HPV 18 in 5 (3.3%) of 150 HR-HPV positive specimens.
As presented in Table IV, two HR-HPV genotypes were observed in 50 (33.3%), three genotypes in 13 (8.7%), four genotypes in 7 (4.7%), and five genotypes in 1 (0.7%) of 150 HR-HPV positive specimens.
| Number of HR-HPV genotypes | ASCUS (N = 20) | LSIL (N = 98) | HSIL (N = 32) | Total HR-HPV positive (N = 150) | ||||
|---|---|---|---|---|---|---|---|---|
| N | Prevalence, % (95% confidence interval) | N | Prevalence, % (95% confidence interval) | N | Prevalence, % (95% confidence interval) | N | Prevalence, % (95% confidence interval) | |
| 2 | 9 | 45 (25.8–65.8) | 33 | 33.7 (25.1–43.5) | 8 | 25 (13–42.3) | 50 | 33.3 (26.3–41.2) |
| 3 | 0 | 0 | 10 | 10.2 (5.5–18) | 3 | 9.4 (2.5–25) | 13 | 8.7 (5–14.4) |
| 4 | 3 | 15 (4.4–36.9) | 4 | 4.1 (1.3–10.4) | 0 | 0 | 7 | 4.7 (2.1–9.5) |
| 5 | 0 | 0 | 1 | 1 (<0.01–6.1) | 0 | 0 | 1 | 0.7 (<0.01–4.1) |
| All infections with multiple HR-HPV | 12 | 60 (38.6–78.2) | 48 | 49 (39.3–58.7) | 11 | 34.4 (20.3–51.8) | 71 | 47.3 (39.5–55.3) |
Of 50 samples with two HR-HPV genotypes, 27 combinations were encountered and 10 appeared more than once. Co-infection with HPV 52 and HPV 58 was the most common combination and appeared seven times, followed by HPV 16 and HPV 51 (five times), HPV 33 and 52 (five times), and HPV 16 and 39 (three times).
Of 13 samples with three HR-HPV genotypes, 12 combinations were detected, 11 appeared only once and co-infection with HPV 16, 52 and 58 appeared twice.
Infections with four genotypes appeared in seven different combinations.
HPV 16 appeared 16 times in combination with other HR-HPV genotypes and was the most frequent individual genotype found in co-infections.
DISCUSSION
In this population-based study cellular abnormalities were found in 202 (3.8%) of 5,300 cervical specimens sent for routine cytological examination by liquid-based Pap smear. These data are consistent with previous findings in the southwestern part of Switzerland and other countries with well-screened populations [Coste et al., 2003; Benard et al., 2004; Petignat et al., 2005; Ronco et al., 2007].
No invasive cervical cancer cases were detected, which could be explained by the fact that the study was performed in a population with a low incidence of cervical carcinoma, ranging between 5.7 and 9.3 per 100,000 women [Petignat et al., 2005].
HR-HPV genotypes were significantly more frequent in patients with high-grade squamous intraepithelial lesions compared to low-grade squamous intraepithelial lesions and specimens with ASCUS.
The current findings are in agreement with the reports from other Western European countries such as Austria, Belgium, France, and Germany. In contrast, no significant difference in the HPV prevalence between high-grade and low-grade lesions was observed in Northern European countries [WHO/ICO Information Centre on HPV and Cervical Cancer, 2007].
In the present study HPV-16 was the most prevalent HR-HPV genotype in all specimens with cytological abnormalities and is comparable with findings worldwide [Clifford et al., 2003, 2005; Smith et al., 2007].
Although presented with lower prevalence in patients with ASCUS and low-grade squamous intraepithelial lesions, HPV 18 became the second most prevalent HR-HPV type in samples with high-grade squamous intraepithelial lesions. It was shown that the distribution of HPV 18 among females with cytological abnormalities differs significantly in Europe and worldwide [Clifford et al., 2003]. In Spain HPV 18 does not appear as common as in this current study [Cobo et al., 2009]. In contrast, HPV 18 was detected in a Greek study in a large proportion of women with cytological abnormalities [Panotopoulou et al., 2007]. Other studies showed that HPV 18 can be under-represented and other high-risk HPV genotypes over-represented in low-grade lesions as well as high-grade lesions in comparison to invasive cervical cancer [Smith et al., 2007].
In addition, HPV 82 which is possibly an HR-HPV with unclear potential was detected only in 0.5% of samples with cytological abnormalities which is comparable with the findings in Germany [Iftner et al., 2010]. Furthermore, HPV 82 was detected only in 0.1% of invasive cervical cancer samples worldwide and was not found in invasive cervical cancer samples in Europe [Smith et al., 2007].
Lower cumulative prevalence of HPV 16 and/or 18 compared to non-HPV 16/18 HR-HPV was seen in patients with ASCUS and low-grade squamous intraepithelial lesions. However, in high-grade squamous intraepithelial lesions HPV 16 and 18 achieved equally high cumulative prevalence as non-HPV 16/18 HR-HPV genotypes. This is in line with other results showing that HPV 16 and HPV 18 become more frequent as individual as well as cumulative genotypes in high-grade dysplasia and cervical cancer cases [Clifford et al., 2005; Khan et al., 2005; Smith et al., 2007; Pannier-Stockman et al., 2008; Wheeler et al., 2009].
Higher prevalence of multiple HR-HPV genotypes in comparison to other studies was observed in all samples with cytological abnormalities [Del Prete et al., 2008; Cobo et al., 2009]. This can be influenced by the demographic factors as well as by the sensitivity of the LA test.
Comparable to other studies, the most frequent multiple infection was associated with two HR-HPV genotypes and was observed in 50 (33.3%) samples and HPV 16 was the most frequent individual genotype found in co-infections [Del Prete et al., 2008; Cobo et al., 2009].
Although single HR-HPV genotypes showed an increasing prevalence from 40% in patients with ASCUS, over 51% in patients with low-grade squamous intraepithelial lesions to 65.6% in patients with high-grade squamous intraepithelial lesions, this difference was not significant.
The LA test, used in this study, is known to be type specific, sensitive and reproducible and was automated recently [Stevens et al., 2007; Dobec et al., 2009; Halfon et al., 2010]. In addition to newly developed high-risk HPV screening tests, such as Digene Hybrid Capture 2, Cervista and Cobas 4800 HPV Test, this assay allows simultaneous detection and typing of 37 different high- and low-risk HPV genotypes.
Current population-based study provides information about the distribution of HPV genotypes in women with cytological abnormalities in the German-speaking part of Switzerland. Future countrywide studies are necessary to determine HPV prevalence, the future impact of vaccines and potential changes in the country's epidemiological HPV profile.
Acknowledgements
This study was performed at Medica, Medizinische Laboratorien Dr. F. Kaeppeli, Zurich, Switzerland and our thanks go to laboratory technicians, Mrs. Liliane Kunz from the Laboratory for molecular diagnostics and Mrs. Britt-Marie Börelius and Mrs. Anja Maurer from the Institute of pathology, for help in performing tests.




