Positive Epstein–Barr virus and cytomegalovirus IgM assays in primary HIV infection
Abstract
We report three cases with misleading cytomegalovirus (CMV) or Epstein–Barr virus (EBV) immunoglobulin M (IgM) results during primary human immunodeficiency virus (HIV) infection. We determined the rate of positive anti-CMV IgM assays or anti-EBV capsid antigen IgM assays in sera from a group of well-characterized subjects with primary HIV infection as 2.9% (1/35; 95%CI: 0.15–16.6%) for each infection. The rate of positive anti-EBV capsid antigen IgM assays in subjects with positive hepatitis A virus IgM assays was 30% (6/20; 95%CI: 14.6–51.9%). Clinicians need to consider the limitations of IgM assays for diagnosis of herpesvirus infections, and consider testing for other infections with overlapping clinical manifestations. J. Med. Virol. 83:1406–1409, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Primary human immunodeficiency virus (HIV) infection is characterized by non-specific clinical features including pyrexia, malaise, rash, pharyngitis, lymphadenopathy, myalgia, and arthralgia [Vanhems et al., 1999]. This presentation resembles the mononucleosis syndrome caused by Epstein–Barr virus (EBV), cytomegalovirus (CMV), and Toxoplasma gondii infections [Hill and Dubey, 2002; Grotto et al., 2003; Taylor, 2003]. The risk of misdiagnosis in primary HIV infection has significant individual and public health consequences. Previous workers have demonstrated the occurrence of positive Monospot tests, positive EBV capsid antigen (EBVCA) immunoglobulin M (IgM) ELISAs (5 of 14 subjects), and EBV nuclear antigen (EBVNA) IgM ELISAs (10 of 14 subjects) in sera from patients with primary HIV infection [Vidrih et al., 2001; Robertson et al., 2003]. The rate of positive EBVCA IgM positivity in cases with acute hepatitis A virus (HAV) infection was also high (12 of 15 subjects) suggesting the possibility of a false positive reaction of the EBVCA IgM assay [Robertson et al., 2003]. We report three cases where positive EBV IgM or CMV IgM results lead to an incorrect diagnosis during primary HIV infection. We then assessed the frequency of positive results that may incorrectly suggest infection with another pathogen in patients with primary HIV infection. We also reassessed the rate of positive EBVCA IgM assays in retrospective serum sample analysis from subjects with positive HAV IgM assays.
METHODS
The cases described were identified from the practice of a single clinician over an 8-year period. None of these subjects were included in the retrospective serum sample analysis.
Eighty-four de-identified serum samples with a positive HIV p24 antigen (p24Ag) test (Vironostika® HIV-1 Antigen, bioMerieux, Boxtel, the Netherlands) and/or indeterminate HIV Western blot (WB) assay (HIV Blot 2.2, MP Diagnostics, St. Ingbert, Germany) using Australian Serology National Reference Laboratory definitions [Healey et al., 1992] were identified at a tertiary referral serology laboratory. All sera were stored at −21°C without repeated thawing. Duplicate samples were excluded, as were samples taken for post-exposure screening in perinatally exposed children; patients with chronic HIV infection; possible false positive cases and samples with insufficient volume for testing. Sera were additionally tested with the HIV BED assay (Calypyte HIV-1 BED Incidence EIA, Calypyte, Portland, ME) to allow exclusion of non-acute HIV infections. Samples that had not been previously tested for HIV p24 Ag were tested for that antigen and all samples with sufficient volume were tested for HIV RNA using the COBAS® Amplicor Ultrasensitive Assay (Roche Diagnostics, Basel, Switzerland) or Versant® HIV-1 RNA 3.0 Assay (Bayer, Berkeley, CA), if not previously tested.
The included sera were tested for monospot reactivity using the Infectious Mononucleosis Kit (Oxoid, Basingstoke, England) and with the EBVCA IgM ELISA (Panbio/Inverness, Windsor, Australia). Anti-EBVCA IgM positive samples were further tested using the EBVCA IgG ELISA (Panbio/Inverness), the EBVNA IgG ELISA (Panbio/Inverness), and the EBVCA IgG avidity assay as described previously [Robertson et al., 2003]. Sera were also tested with an anti-CMV IgM ELISA (ETI-CYTOK-M Reverse PLUS, DiaSorin, Saluggia, Italy). Positive samples were tested with an anti-CMV IgG ELISA (ETI-CYTOK-G PLUS, DiaSorin) and positive samples were tested for CMV IgG avidity as previously described [Munro et al., 2005]. Anti-CMV IgM or anti-EBV IgM-positive samples were tested with an in-house nested multiplex PCR (mPCR) for EBV and CMV [McIver et al., 2005]. Eighteen samples were tested using the Merifluor TOXO IgM Immunofluoresence Assay (IFA; Gull Laboratory, Salt lake City, UT) and the remaining 17 using the Toxo-Spot IFA (bioMérieux, Mercy l'Etoile, France) due to changes in kit availability. Equivocal results were retested using the automated AxSYM Toxo IgM assay (Abbott Diagnostics, Weisbaden, Germany). All samples were tested with a Parvovirus B19 IgM ELISA (Biotrin International, Dublin, Ireland). Additionally, 20 de-identified sera with reactive hepatitis A IgM assay results (Abbott Architect, Abbott Diagnostics) were tested with the above-mentioned serological assays for EBV infection.
The study was approved by the Human Research Ethics Committees of the University of New South Wales and South Eastern Sydney and Illawarra Area Health Service.
RESULTS
Case 1
A 30-year-old woman with fever, rash, diarrhea, lymphadenopathy, and biochemical hepatitis was reviewed at an emergency department where testing for hepatitis C virus (HCV) and hepatitis B virus (HBV) infection was negative. She subsequently had persistent lymphadenopathy for 7 months. A fine needle biopsy of a cervical lymph node revealed reactive changes. Testing for both CMV IgM and CMV IgG were positive. A hematologist made a provisional diagnosis of CMV infection. HIV infection was confirmed serologically 6 months later when she presented with genital herpes.
Case 2
A 20-year-old man with fevers, night sweats, diarrhea, headache, and vomiting was reviewed in the emergency department where examination revealed stomatitis, gingvival erythema, and cervical lymphadenopathy. Investigations revealed pancytopenia. The tests for both CMV IgM and CMV IgG were positive and were considered diagnostic. Initial HIV antibody testing was negative. He was subsequently reviewed by the infectious diseases team and repeat HIV testing was undertaken as he reported male to male sexual activity. One week later the HIV p24Ag and the HIV antibody assay were positive with an indeterminate HIV WB.
Case 3
A 25-year-old man developed prolonged illness with fever, rash, lethargy, sore throat, poor appetite, and weight loss. He was tested for EBV infection and results of both IgM and IgG testing were positive. He was told he had glandular fever. He did not feel that he could disclose that he had sex with both men and women and was not tested for HIV at this time. He sought HIV testing 2 months later and HIV was confirmed serologically at that time.
Retrospective Sample Review
Of the 84 initial HIV serum samples, 49 were excluded (see Fig. 1). Of the 14 samples that were considered false-positive HIV ELISA assays, 2 out of 14 were from pregnant subjects with a negative proviral HIV DNA assay, 2/14 subjects had an unchanging HIV WB in follow-up, 1/14 subjects had a subsequent negative HIV WB after an initial indeterminate HIV WB, and 9/14 subjects had undetectable p24Ag and viral load assays. Thirty-five samples from patients with primary HIV infection were available for testing. The characteristics of the samples and the results of testing are shown in Figure 1. Of the 35 samples, 1/35 (2.9%; 95%CI: 0.15–16.6%) was positive for anti-EBVCA IgM, anti-EBVCA IgG, and anti-EBVNA IgG, with high anti-EBVCA IgG avidity. There was also 1/35 positive (2.9%; 95%CI: 0.15–16.6%) sample in the anti-CMV IgM ELISA that was also positive for anti-CMV IgG with high anti-CMV IgG avidity. The serum mPCR assay did not detect viral nucleic acid of CMV or EBV in either sample. These results are consistent with the positive IgM assay in each case not being associated with acute EBV or CMV coinfection.
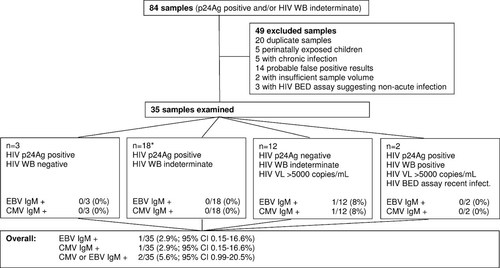
Serum sample selection and key results. p24Ag, HIV p24 antigen; HIV WB, HIV Western blot; HIV VL, HIV viral load. *Four of the samples in this group did not have sufficient sample for HIV viral load testing and all but two samples had sufficient sample volume for HIV BED assay testing.
All samples tested negative using the Monospot, T. gondii IFA, and anti-Parvovirus B19 IgM ELISA, with 4/35 equivocal on the Toxo-Spot IF kit which were subsequently negative when tested with the AxSYM Toxo IgM assay.
Overall, there were 2/35 (5.7%; 95%CI: 0.99–20.5%) samples positive for IgM assays for infections that may be confused with primary HIV infection.
There were 6/20 (30%; 95%CI: 14.6–51.9%) HAV IgM reactive samples were reactive with EBV IgM and all EBV IgM reactive samples were reactive for EBV VCA IgG and EBV EBNA IgG assays and had EBV avidity ranging from 52% to 93%.
DISCUSSION
The findings from this study confirm other studies suggesting that acute primary HIV infection may be associated with positive IgM assays against common herpesviridae that have an clinical presentation overlapping with that of primary HIV infection [Robertson et al., 2003]. The data in the present study are consistent with a rate of 1–20% false positivity for CMV or EBV IgM in primary HIV infection. The rate of EBV IgM positivity is significantly lower with the present assay systems than found in a previous study [Robertson et al., 2003], and also lower in samples reactive for HAV IgM (30% vs. 80%). This may be due to improved specificity of the assay system.
It is unlikely that the samples with positive IgM assays represented recent coinfection with EBV or CMV as the samples were IgG positive with a high IgG avidity and negative on mPCR assay, consistent with past infection [Munro et al., 2005]. This strongly suggests very recent primary infection or viral reactivation was not the cause of the observed serologic patterns. It is possible, however, that these results represent persistent IgM reactivity from natural infection months to years previously.
Interestingly, the reactive samples on the herpesviridae IgM assays shared the same HIV serologic characteristics of a negative HIV p24Ag assay and indeterminate HIV WB. This suggests that false-positive results may occur when the p24Ag has already fallen to an undetectable level during the period of the developing HIV-specific antibody response [Parry, 2002].
This study had several limitations. It was a retrospective study and detailed clinical information was not available on all subjects. However, all sera were likely to represent acute primary infections given the available clinical and laboratory data. The sera were stored for up to 5 years at −20°C, and were not subject to repeated freeze–thaw cycles. The sample size was limited and further studies are needed to confirm the frequency of serological misdiagnosis in acute HIV infection.
Clinicians should consider that primary HIV infection may be associated with positive herpesviridae IgM assays. Clinicians should consider testing for primary HIV infection in cases where a serological diagnosis of CMV or EBV is based predominantly on the reactivity of an IgM assay or when the antibody avidity against EBV or CMV is high.
Acknowledgements
Funding for the serological testing was provided as part of the Independent Learning Project funding for Mun Khin Chan's project as a medical student at the University of New South Wales.




