Interference of replication between hepatitis B and C viruses in patients infected with HIV†
The authors have no commercial or other association that might pose a conflict of interest.
Abstract
The clinical and cellular interactions between hepatitis B virus (HBV) and hepatitis C virus (HCV) were investigated in patients co-infected with the human immunodeficiency virus (HIV). One hundred ninety-nine patients followed for 6 years were evaluated to compare the level of HBV DNA and HCV RNA in patients co-infected with HIV and HBV, and patients co-infected with HIV, HBV, and HCV. A full-length HBV genome and HCV JFH1 RNA were co-transfected into HuH-7.5.1 cells in vitro to examine the impact of co-infection and dependence on the HBV PreC mutant for replication interference. Before 2′,3′-dideoxy-3′-thiacytidine (3TC)-based antiretroviral therapy (ART) was initiated, HBV DNA was found in 56/123 (45.4%) patients co-infected with HIV and HBV, and in 19/76 (25.0%) patients co-infected with HIV, HBV, and HCV. After 3TC-based ART was initiated, detectable HBV DNA decreased to 7/76 (9.2%) in patients co-infected with HIV, HBV, and HCV, but HCV RNA increased from 43/76 (56.6%) to 60/76 (78.9%) (P = 0.003). In vitro HBV and HCV co-infection led to decreased replication of both viruses. The primary factors that influenced the decreased replication were the order of the HBV and HCV infection and the HBV PreC mutation. J. Med. Virol. 83:1159–1164, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Although co-infection with the human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) is common in commercial plasma donors, some blood recipients, and intravenous drug users [Rong-Rong et al., 2008], the influence of HBV/HCV co-infection on the level of HBV DNA and HCV RNA is a controversial issue [Sagnelli et al., 2000, 2004, 2006; Bonacini et al., 2004; Liu and Hou, 2006]. In the present study, the clinical characteristics of patients co-infected with HIV and HBV, or HIV, HBV and HCV, were compared during a 6-year follow-up period. To evaluate interference in virus replication during co-infection with HBV and HCV, a transient-transfection method was used by co-transfecting the full-length HBV genome with HCV JFH1 into the HuH-7.5.1 human hepatoma cell line.
MATERIALS AND METHODS
Patients
Four acquired immunodeficiency syndrome (AIDS) hospital units in different geographical areas of China participated in the study: one in Northwest China, two in Central China, and one in Southern China. These AIDS units have participated in numerous multicenter studies on AIDS in the past 10 years. Criteria for the clinical approach and diagnosis throughout followed the National Free ART Guideline [Zhang, 2005].
Criteria for inclusion were (1) age ≥18 years, (2) a known route of HIV infection, (3) positive for hepatitis B surface antigen (HBsAg) for at least 6 months, (4) naïve for anti-HBV treatment at enrollment, and (5) months on follow-up ≥18 months. From January 2002 through December 2009, a total of 2,361 HIV-infected subjects (mean age was 35.3 ± 4.7 years, ranging from 18 to 60 years) were detected for HBsAg and anti-HCV antibody. Two hundred fifty-five were tested positive for HBsAg (10.8%), of whom, 56 (22.0%) were excluded because they had HBsAg positivity for <6 months, or they were followed-up for <18 months. A total of 199 HIV-infected subjects, positive for HBsAg, were enrolled in this study. They were sub-categorized into two case types: 123 patients who were positive for HBsAg but negative for anti-HCV antibody and HCV RNA (Case Group HIV/HBV); and 76 patients who were positive for HBsAg and anti-HCV antibody (Case Group HIV/HBV/HCV).
All the patients in this study received 2′,3′-dideoxy-3′-thiacytidine (3TC; lamivudine) at a dose of 300 mg/day. Plasma samples were tested for HBV DNA and HCV RNA at enrollment, and then every 3–6 months during subsequent follow-up visit. All these samples were stored at −80°C, and were not thawed until used for this investigation.
Ethical Approval
This study was approved by the ethics committee of Zhongnan Hospital of Wuhan University. Informed consents on the collection of demographic data and plasma samples were obtained from all patients. All experiments were performed in accordance with the ethical standards of the Declaration of Helsinki.
Diagnostic Testing for HBV Markers and Anti-HCV Antibody
Enzyme immunoassay kits from the Shanghai Kehua Biology Company were used to test for HBsAg, hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg), hepatitis B e antibody (HBeAb), hepatitis B core antibody (HBcAb), and anti-HCV antibody. Sample evaluation was performed strictly according to the manufacturer's instructions.
Quantitative Detection of HBV DNA and HCV RNA in Plasma or Supernatant by Real-Time PCR
Real-time PCR kits from the Shanghai Kehua Biology Company were used to test for HBV DNA and HCV RNA levels according to the manufacturer's instructions. The detection limit of the real-time PCR for HBV DNA and HCV RNA was 500 genome copies/ml and 103 IU/ml, respectively.
YMDD Mutation Analysis
YMDD mutation was determined as described previously [Xu et al., 2010].
Liver Function Tests
Liver function was assessed using an automatic biochemical analyzer manufactured by the Rili Company (Tokyo, Japan).
Cell Line, Plasmids, and HCV JFH1
The human hepatoma Huh 7.5.1 cell line was used for HBV genome (a DNA plasmid) and HCV genome (RNA) transfection. Plasmids that contained a 1.3-fold full-length HBV wild-type (WT) or PreC mutant (1896) genome were constructed in the State Key Laboratory of Virology, College of Life Science, Wuhan University. The Japanese fulminate hepatitis 1 (pJFH1) plasmid was provided by T. Wakita (National Institute of Infectious Diseases, Tokyo, Japan). The green fluorescent protein (GFP) plasmid was used to determine the transfection efficiency for each experiment.
Transfection
To assess the effects of HBV and HCV co-infection in vitro, a cell culture model of co-infection using the Huh 7.5.1 cell line was established. To determine the transfection efficiency, 8 × 104 cells were seeded into 24-well plates and transfected 24 hr later with 1.0 µg of in vitro-transcribed mRNA that encoded GFP. Liposome-mediated transfection using Lipofectamine 2000 reagent (Invitrogen Life Technologies, Carlsbad, CA) was used for all transfection experiments. To determine if HBV and HCV replication cycles interfered with one another, the HBV 1.3-fold genomic DNA and HCV JFH1 RNA were transfected into Huh 7.5.1 cells either in sequence (HBV then HCV or HCV then HBV) or simultaneously. For HCV infection subsequent to HBV infection, HCV-JFH1 RNA was transfected at 6–8 hr after transfection with the HBV 1.3-fold genomic DNA. For HBV infection subsequent to HCV infection, the HBV 1.3-fold genomic DNA was transfected at 4–6 hr after HCV-JFH1 RNA. For concurrent HBV/HCV infection, HBV 1.3-fold genomic DNA and HCV-JFH1 RNA were co-transfected. In each case, fresh medium was replaced 24 hr later. Viral nucleic acid replication was assessed 2 days post-transfection from cell culture supernatants.
Statistical Analysis
Fisher's two-tailed exact test and the χ2 test were used to compare qualitative data. The statistical package for social sciences (SPSS), version 13.0, was used for all statistical analyses. Significance was set at P < 0.05.
RESULTS
Demographic and Clinical Characteristics of Patients
Among the 123 patients co-infected with HIV and HBV, and 76 patients co-infected with HIV, HBV, and HCV, 15 of 123 (12.2%) and 9 of 76 (11.8%), respectively, were positive for HBeAg. The remaining 175 individuals (87.9%) were negative for HBeAg. Patients in Case Group HIV/HBV/HCV and Case Group HIV/HBV were comparable with regard to sex, age, rate of abnormal liver function tests, and baseline CD4 cell counts (Table I).
| HIV/HBV group (n = 123) | HIV/HBV/HCV group (n = 76) | P-value | All patients (n = 199) | |
|---|---|---|---|---|
| Age at enrollment, median years (IQR) | 34.8 (29–41) | 36.2 (30–43) | 0.771 | 35.2 (30–42) |
| Male | 66 (53.7) | 45 (59.2) | 0.444 | 111 (55.8) |
| HIV transmission route | ||||
| Commercial plasma donationa | 21 (17.1) | 37 (48.7) | <0.05 | 58 (29.1) |
| Blood transfusion | 22 (17.9) | 19 (25.0) | 0.228 | 41 (20.6) |
| Injection drug use | 7 (5.7) | 18 (23.7) | <0.05 | 25 (12.6) |
| Heterosexual | 60 (48.8) | 1 (1.3) | <0.05 | 61 (30.7) |
| Homosexual | 13 (10.6) | 1 (1.3) | <0.05 | 14 (7.0) |
| Baseline CD4 cell count, median cells/µl (IQR) | 114 (40–178) | 102 (36–155) | 0.920 | 107 (38–160) |
| ALT elevation at baseline, U/L | 14 (11.4) | 7 (9.2) | 0.628 | 21 (10.6) |
| HBeAg+ at baseline | 15 (12.2) | 9 (11.8) | 0.941 | 24 (12.1) |
| Median follow-up time, months (IQR) | 72.3 (18–82) | 72.7 (18–86) | 0.937 | 72.5 (17–84) |
- IQR, interquartile range; ALT, alanine aminotransferase.
- Data are no. (%) of patients, unless otherwise indicated.
- a In central China during the 1990s, an array of commercial plasma/blood collection stations, where individuals were paid to donate plasma/blood, pooled and centrifuged the blood, retained the plasma, and re-infused blood cells into donors using unsanitary medical equipment caused a rapid spread of HIV and HCV [Qian et al., 2006; Meade et al., 2010].
CD4 Cell Counts, Viral DNA/RNA, and YMDD Mutation Fluctuation During the Follow-Up
The longer time of 3TC-based antiretroviral therapy (ART) was accompanied by increases in HBV DNA and YMDD mutation, while CD4 cell counts increased during 36 months but decreased after 36 months. HBV DNA changed in parallel with fluctuations of the YMDD mutation. The results are shown in Table II.
| Follow-up time (months) | HIV/HBV | HIV/HBV/HCV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tested number | CD4 (cells/µl) mean ± SD | HBV DNA+ (n %) | YMDD mutation (n %) | Tested number | CD4 (cells/µl) mean ± SD | HBV DNA+ (n %) | YMDD mutation (n %) | HCV RNA+ (n %) | |
| 12 | 123 | 267.0 ± 12.3 | 5 (4.1) | 3 (2.4) | 76 | 247.7 ± 10.4 | 1 (1.3) | 0 (0) | 69 (90.8) |
| 24 | 86 | 322.7 ± 27.4 | 14 (16.3) | 13 (15.1) | 72 | 330.2 ± 36.5 | 5 (6.9) | 4 (5.6) | 62 (86.1) |
| 36 | 72 | 381.4 ± 19.7 | 23 (31.9) | 20 (27.8) | 58 | 375.3 ± 25.6 | 10 (17.2) | 10 (17.2) | 51 (87.9) |
| 48 | 50 | 313.8 ± 30.4 | 28 (56.0) | 27 (54.0) | 39 | 302.6 ± 30.8 | 14 (35.9) | 12 (30.8) | 32 (82.1) |
| 60 | 32 | 224.6 ± 41.5 | 18 (56.3) | 18 (56.3) | 19 | 245.0 ± 45.7 | 7 (36.8) | 7 (36.8) | 16 (84.2) |
| 72 | 20 | 189.0 ± 36.3 | 13 (65.0) | 13 (65.0) | 18 | 201.5 ± 23.1 | 9 (50.0) | 9 (50.0) | 16 (88.9) |
| P-value | — | <0.05 | <0.05 | <0.05 | — | <0.05 | <0.05 | <0.05 | 0.834 |
Effect of ART With 3TC on the Positive Rates and Mean Concentration of HBV DNA and HCV RNA
Before ART was initiated, serum HBV DNA was detected in 19/76 (25%) patients in Case Group HIV/HBV/HCV, whereas in 56/123 (45.5%) patients in Case Group HIV/HBV. After 3TC-based ART was initiated, the number of individuals who had detectable HBV DNA dramatically decreased both in Case Group HIV/HBV and Case Group HIV/HBV/HCV (Table III). In contrast, the number of individuals who had detectable HCV RNA increased in Case Group HIV/HBV/HCV (P = 0.003).
| HIV/HBV group (n = 123) | HIV/HBV/HCV group (n = 76) | |||||
|---|---|---|---|---|---|---|
| Pre-ART | At the last observation | P-value | Pre-ART | At the last observation | P-value | |
| HBV DNA+ | ||||||
| Number of patients with detectable HBV DNA (%) | 56 (45.5) | 19 (15.5) | <0.05 | 19 (25.0) | 7 (9.2) | <0.05 |
| Mean concentration of HBV DNA (mean ± SD, log10 copies/ml)a | 6.01 ± 0.59 | 4.25 ± 1.16 | <0.05 | 5.59 ± 0.55 | 4.07 ± 1.01 | <0.05 |
| HCV RNA+ | ||||||
| Number of patients with detectable HCV RNA (%) | — | — | — | 43 (56.6) | 60 (78.9) | <0.05 |
| Mean concentration of HCV RNA (mean ± SD, log10 IU/ml)a | — | — | — | 4.48 ± 1.11 | 5.83 ± 1.04 | <0.05 |
- a These data were analyzed from HBV DNA or HCV RNA positive patients. Values are expressed as the mean ± standard deviation.
The Relation Between HBV DNA and Disease Progression
The incidence of hepatic decompensation and cirrhosis of the liver in patients who had undetectable HBV DNA was much lower, compared with in those who had detectable HBV DNA (Table IV).
| Disease progression | HIV/HBV group | HIV/HBV/HCV group | ||||
|---|---|---|---|---|---|---|
| HBV DNA+ (n = 56) | HBV DNA− (n = 67) | P-value | HBV DNA+ (n = 19) | HBV DNA− (n = 57) | P-value | |
| Hepatic decompensation | 6 (10.7) | 0 (0) | <0.05 | 1 (5.3) | 0 (0) | <0.05 |
| Liver cirrhosis | 19 (33.9) | 3 (4.5) | <0.05 | 2 (10.5) | 0 (0) | <0.05 |
| Hepatocellular carcinoma | 5 (8.9) | 3 (4.5) | 0.319 | 3 (15.8) | 0 (0) | <0.05 |
- Data are no. (%) of patients.
Detection of HBsAg and HBeAg in Cell Culture Supernatants After HBV Genomic DNA Transfection
HBsAg and HBeAg were both detectable in the supernatant of HBV WT transfected cells, but only HBsAg was detectable in the supernatant of cells transfected with the 1896 PreC mutant. Neither HBsAg nor HBeAg was detectable in the supernatant of cells that were transfected with the pBlueScript negative control plasmid. The assays demonstrated that these plasmids supported HBV replication and protein expression as expected.
Evaluation of HBV DNA and HCV RNA in Huh 7.5.1 Cell Culture Supernatant During Subsequent and Concurrent Infections
Interference was determined by a decrease in release of HBV genomic DNA or HCV genomic RNA, which represented new virus synthesis. It was found that HBV and HCV did interfere with each other; however, interference of viral replication only occurred between the HBV PreC mutant (1896) and HCV when HCV RNA was transfected into cells after HBV infection was established (Fig. 1a,b).
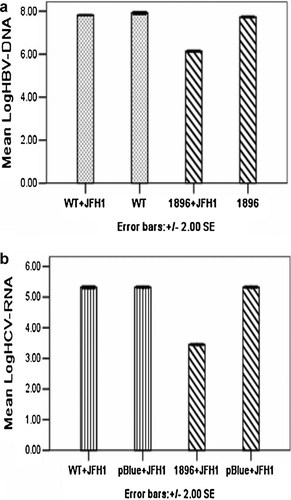
a: Log HBV DNA in supernatant when HCV JFH1 was added 6–8 hr after HBV plasmid transfection. b: Log HCV RNA in supernatant when HCV JFH1 was added 6–8 hr after HBV plasmid transfection. WT + JFH1: transfection with HBV wild-type plasmid DNA and HCV JFH1 RNA; WT: transfection with HBV wild-type plasmid DNA alone; 1896 + JFH1: transfection with HBV 1896 PreC mutant plasmid DNA and HCV JFH1 RNA; 1896: transfection with HBV 1896 PreC mutant plasmid DNA alone; pBlue + JFH1: transfection with pBlueScript SK plasmid DNA and HCV JFH1 RNA.
No interference was observed when HBV DNA was transfected into cells after HCV RNA.
When HBV 1.3-fold genomic DNA and HCV JFH1 RNA were transfected simultaneously into Huh 7.5.1 cells, interference of viral replication only occurred between the HBV WT and HCV (Fig. 2a,b).
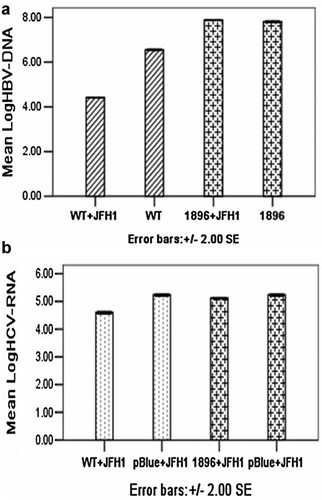
a: Log HBV DNA in supernatant when HBV 1.3-fold genomic DNA and HCV JFH1 RNA were transfected simultaneously into Huh 7.5.1 cells. b: Log HCV RNA in supernatant when HBV 1.3-fold genomic DNA and HCV JFH1 RNA were transfected simultaneously into Huh 7.5.1 cells. WT + JFH1: transfection with HBV wild-type plasmid DNA and HCV JFH1 RNA; WT: transfection with HBV wild-type plasmid DNA alone; 1896 + JFH1: transfection with HBV 1896 PreC mutant plasmid DNA and HCV JFH1 RNA; 1896: transfection with HBV 1896 PreC mutant plasmid DNA alone; pBlue + JFH1: transfection with pBlueScript SK plasmid DNA and HCV JFH1 RNA.
DISCUSSION
This study is believed to be the first in China to show clinical and cellular evidence of an interaction between HBV and HCV infection among individuals co-infected with HIV. Viral kinetics of HBV and HCV indicated reciprocal changes. The significant decrease in the detection of serum HBV DNA and lower concentration of HBV DNA levels among the patients co-infected with HIV, HBV, and HCV compared to those co-infected with HIV and HBV demonstrated interference in the replication cycles between HBV and HCV.
The effectiveness of 3TC for the treatment of chronic hepatitis B is well documented, providing significant reductions in serum HBV DNA. Among the 76 patients co-infected with HIV, HBV, and HCV (Case Group HIV/HBV/HCV), serum HBV DNA was detected in 19 patients before ART and in 7 patients after 3TC-based ART. The proportion of patients with serum HBV-DNA changed from positive to negative was 63.2%. These data confirmed that 3TC is able to inhibit HBV replication. In contrast, HCV viremia was detectable in 78.9% patients co-infected with HIV, HBV, and HCV who were treated with 3TC, but in only 56.6% of ART naïve patients. Thus, although 3TC decreased HBV DNA levels in patients co-infected with HIV, HBV, and HCV, the replication of HCV was increased. These results are similar to those of Filippini et al. [2007], who also found that HCV viremia in patients with HIV infection increased when 3TC-containing ART was initiated. Similarly, in a study by Chuang et al. [2005] in patients with HBV/HCV co-infection, treatment with six MU IFN-α-2b three times weekly plus 1,000–1,200 mg/day ribavirin for 24 weeks has demonstrated that, among those who responded to HCV antiviral therapy, there was a significantly higher rate of HBV DNA in the blood than among the HCV non-responders during and after treatment. These data indicate that during 3TC therapy for HBV/HCV co-infection, suppression of these viruses can result in rebound of HCV RNA. These data support the hypothesis that there might be reciprocal viral interference between HBV and HCV in HIV co-infected patients, and that anti-HCV or anti-HBV therapy might increase replication of the untreated co-infecting hepatitis virus.
HBV replication is associated with liver damage and subsequent disease progression, including hepatic decompensation and development of liver cirrhosis or hepatocellular carcinoma (HCC). For the patients co-infected with HIV and HBV, or HIV, HBV, and HCV, especially those with detectable HBV DNA, 3TC-based ART regimen might suppress the replication of HBV and result in a lower incidence of end stage liver disease.
In the present study, both sequential and concurrent HBV/HCV infection of hepatocytes by transfection of Huh 7.5.1 cells with plasmid that encoded a 1.3-fold HBV WT or 1896 PreC mutant genome and HCV JFH1 RNA were mimicked. For the sequential transfection, both orders, that is, HBV then HCV and HCV then HBV were tested. When HCV RNA was introduced after HBV 1896 DNA (HCV superinfection of HBV-infected cells), the amount of detectable HBV DNA release into the cell culture supernatant was reduced compared to HBV 1896 alone, HBV WT alone, and HBV WT followed by HCV. In contrast, introduction of HBV DNA into cells infected with HCV showed no replication interference with either virus. Thus, these data indicate that HCV interferes with the HBV PreC mutant but not with HBV WT replication, but the HCV infection has to follow HBV infection. The results of these in vitro studies correlate with clinical data that have shown a reduction in serum HBV DNA among patients co-infected with HIV, HBV, and HCV. Finally, during concurrent HBV/HCV infection, a reduction in HBV DNA and HCV RNA was observed in cells co-transfected with HBV WT but not HBV 1896 DNA.
The molecular mechanisms responsible for the suppressive effect of dual HBV/HCV infection remain unclear. Other in vitro studies have demonstrated that HCV suppression of HBV replication is mediated by HCV core protein [Shih et al., 1995; Schuttler et al., 2002; Chen et al., 2003]. One study has reported that HCV core protein suppresses HBV replication by inhibition of transcription and encapsidation of the HBV pregenomic RNA [Shih et al., 1993]. HBV replication is governed by four promoters and two enhancers, enhancer 1 (Enh1) and enhancer 2 (Enh2) [Williams et al., 2009]. The Enh2 domain is an important determinant of promoter activity in vivo [Williams et al., 2009]. HCV core protein suppresses HBV Enh1 and Enh2 11- and 4-fold, respectively [Chen et al., 2003]. However, the underlying molecular mechanisms responsible for this suppressive effect require further study.
The reciprocal inhibitory effect between HBV and HCV suggest that patients who are triple-infected with HIV, HBV, and HCV face new challenges during ART. HIV-infected individuals, especially those prepared to start ART, should be tested for HBV and HCV so as to select drugs that will not disturb this co-inhibitory effect, but will compliment potential HBV or HCV antiviral treatments. It is necessary to determine serum HBV DNA and HCV RNA levels regularly for patients co-infected with HIV, HBV, and HCV during ART, and clinical diagnostic measures of liver injury should be followed carefully. During treatment of patients who are co-infected with HBV and HCV, physicians should be aware that antiviral treatment for only one infection could exacerbate hepatitis caused by the second virus, by removing inhibitory factors. Such complications could be circumvented by the development of new antiviral drugs that are effective against both HBV and HCV.
There were some limitations in this study. In addition to 3TC, other agents with both anti-HIV and anti-HBV activity, such as emtricitabine and tenofovir (FTC and TDF), are unavailable as first-line drugs in China. Information about the effect of FTC- or TDF-based ART on HBV DNA levels was unobtainable. The correlation between HBV DNA positivity, CD4 cell count, and the time of ART are important to evaluate the effect of 3TC-based ART. Further study will focus on the differences of the emergence in time of YMDD motif mutation between patients co-infected with HIV and HBV, and those co-infected with HIV, HBV, and HCV, during 3TC-based ART.
Acknowledgements
We thank Yiqun Gui for providing suggestions and English translation. We also thank Yinpu Yue for technical assistance. We thank all of the clinicians for providing samples and clinical data from the patients. The English in this document has been checked by at least two professional English editors.




