Correlation of serum hepatitis B surface antigen level with response to entecavir in naïve patients with chronic hepatitis B
Abstract
Recent studies have suggested that quantifying the serum HBsAg levels can predict the response to pegylated interferon. We aimed to determine the change in serum HBsAg levels during entecavir (ETV) treatment and the correlation with treatment response in chronic HBeAg-positive and HBeAg-negative hepatitis B patients. Serial HBsAg levels were measured using the Architect assay (Abbott Laboratories, Abbott Park, IL) in sera from 101 treatment-naive chronic hepatitis B (CHB) patients receiving ETV. During treatment, in HBeAg-positive patients, the mean HBsAg level was 3.51, 3.22, 3.34, 3.36, and 3.40 log10 IU/ml at baseline, 3, 6, 12, and 24 months, respectively, and there was no significant change compared with the baseline level, except the decline at 3 months (P = 0.009). In HBeAg-negative patients, the mean level of serum HBsAg showed increase with 3.06, 3.09, 3.20, 3.26, and 3.27 log10 IU/ml at baseline, 3, 6, 12, and 24 months of treatment, respectively. In HBeAg-positive patients, HBV-DNA negativity (<2,000 copies/ml; P = 0.010) and HBsAg level <3,000 IU/ml (P = 0.026) at 3 months were independent predictors of HBeAg loss/seroconversion at 12 months. After 24 months of treatment, the HBsAg levels at baseline (P = 0.046) was an independent factor of HBeAg loss/seroconversion. In HBeAg-negative patients, undetectable HBV DNA at 6 months was an independent factor predicting undetectable HBV DNA after 12 months of therapy. The level of serum HBsAg before and during therapy was a good predictor of HBeAg loss/seroconversion in naïve HBeAg-positive CHB patients receiving entecavir. J. Med. Virol. 83:1178–1186, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
The treatment of patients with chronic hepatitis B (CHB) has evolved during the past 5 years. However, much remains to be accomplished because current drug therapy has limited success in achieving durable end-points and antiviral resistance may emerge during long-term therapy [Andersson and Chung, 2009]. Therefore, monitoring the virologic response during and after treatment is essential. Monitoring the serum hepatitis B antigen (HBeAg) [Yuen et al., 2007; Gauthier et al., 1999] and hepatitis B virus (HBV)-DNA levels [Mommeja-Marin et al., 2003; Chan et al., 2008] is used to assess the response to antiviral treatment, achievement the treatment end-point, and detection the emergence of antiviral resistance. However, these methods have some problems, such as the presence of HBeAg-negative variants and the high cost or cumbersome procedures for assay of serum HBV-DNA [Ozaras et al., 2008].
Recently, a quantitative assay of the serum HBsAg level has been reported as a useful method for monitoring the antiviral response. Serum HBsAg levels are strongly correlated with intrahepatic HBV DNA and covalently closed circular DNA (cccDNA); cccDNA is superior to serum HBV DNA as a predictor of the sustained response to antiviral therapy [Sung et al., 2005; Wursthorn et al., 2006; Chan et al., 2007]. Specifically, in patients with HBeAg-negative CHB treated with pegylated interferon, the on-treatment decline in the HBsAg level was significantly associated with sustained HBsAg clearance 3 years after treatment [Brunetto et al., 2009]. However, lamivudine had little effect on HBsAg levels despite lowering the HBV-DNA level [Brunetto et al., 2009].
During treatment with entecavir (ETV), one of the potent oral cyclopentyl guanosine analogs, limited information is available on the relationship between the HBsAg level and treatment response. A significant association between the HBsAg level and the response to ETV has recently been reported in a small number of HBeAg-positive patients [Jung et al., 2010].
Therefore, the aim of the present study was to evaluate the change in the serum HBsAg level during ETV treatment and the correlation with treatment response in naïve CHB patients, and to compare HBsAg level as a predictor of response between in HBeAg-positive and -negative patients.
MATERIALS AND METHODS
Patients
One hundred and one treatment–naïve patients with CHB were consecutively enrolled between September 2006 and July 2009 from Ajou University Hospital and served as the study subjects. All of the patients received 0.5 mg ETV once daily for >12 months, and 58 patients continued treatment for >24 months. All patients were positive for HBsAg and negative for anti-HBsAg antibody for at least 6 months. Patients who had hepatocellular carcinoma on presentation or other concomitant diseases, including hepatitis C, autoimmune hepatitis, and alcoholic liver disease, were excluded. Serum samples were obtained at baseline and every 3 months during the administration of ETV. Treatment response was defined as serum HBV-DNA levels undetectable or HBeAg loss/seroconversion in HBeAg-positive patients, and serum HBV-DNA levels undetectable in HBeAg-negative patients.
The study protocol was approved by the Institutional Review Board for Human Research at Ajou University Hospital. Informed consent to participate in the study was obtained from all of the study subjects.
Biochemical and Serologic Tests
Serum biochemical parameters, including total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), albumin, blood urea nitrogen, creatinine, α-fetoprotein (AFP), prothrombin time, blood glucose, triglycerides, and total cholesterol, were measured using standard procedures. The hepatitis B virus markers included HBsAg, anti-HBc, anti-HBs, HBeAg, and anti-HBe (Abbot Laboratories, Chicago, IL). Serum HBV DNA was quantified using the b-DNA assay (Versant™ 3.0, lower limit of detection, 2,000 copies/ml; Bayer Healthcare LLC Diagnostic Division, NY) until December 2008, after which a PCR assay (lower limit of detection, 50 copies/ml; Roche Diagnostics, Branchburg, NJ) was used. Samples with HBV-DNA levels <2,000 copies/ml using the b-DNA assay at 6 and 12 months of treatment were retested using the COBAS TaqMan™ PCR assay (TaqMan test; Roche Diagnostics, Branchburg, NJ).
HBsAg was quantified using the Architect HBsAg assay (dynamic range, 0.05–250.0 IU/ml; Abbott Laboratories, Abbott Park, IL). Samples with HBsAg levels >250.0 IU/ml were retested after diluting 1:500.
Statistical Analyses
The patient characteristics are given as the mean ± SD, as appropriate. The variables that differed between the patients with and without a treatment response were identified by Fisher's exact tests and independent t-test analyses. To identify the independent factors, the variables that were significant on univariate analysis (P < 0.05) were subjected to multivariate analysis using the logistic regression model with stepwise forward selection. The Pearson (γ) and bivariate Spearman (σ) rank correlation coefficient were used for measurement of the relationship between HBsAg and baseline factors. Statistical analyses were performed with SPSS 12.0 (SPSS, Inc., Chicago, IL).
RESULTS
Baseline Characteristics
The study population comprised 59 HBeAg-positive and 42 HBeAg-negative patients. The baseline characteristics of the 101 patients are shown in Table I. The mean age was 41 and 47 years in HBeAg-positive and -negative patients, respectively. The mean value of serum ALT was 227.9 and 163.2 IU/L in HBeAg-positive and -negative patients, and AST was 150.0 and 111.1 IU/L, respectively. The mean level of serum HBsAg was 3.51 and 3.06 log10 IU/ml in HBeAg-positive and -negative patients, respectively.
| HBe (+) patients (n = 59) | HBe (−) patients (n = 42) | |
|---|---|---|
| Mean age, years (SD) | 41.3 (±9.57) | 46.6 (±8.49) |
| Male, n (%) | 44 (74.6) | 30 (71.4) |
| Mean HBsAg, log10 IU/ml (SD) | 3.51 (±1.06) | 3.06 (±0.69) |
| Mean ALT, IU/L (SD) | 227.90 (±274.31) | 163.21 (±121.89) |
| Mean AST, IU/L (SD) | 150.00 (±201.15) | 111.07 (±84.25) |
| Mean GGT, (SD) | 72.58 (±45.33) | 62.07 (±33.01) |
| Mean duration of entecavir, months (SD) | 21.3 (±4.88) | 22.3 (±4.57) |
| HBV DNA, log10copies/ml (n, %) | ||
| >8 | 38 (64.4) | 12 (28.6) |
| ≤8 | 21 (35.6) | 30 (71.4) |
| Liver cirrhosis, n (%) | 11 (18.6) | 20 (47.6) |
| Viral breakthrough, n (%) | 0 (0) | 0 (0) |
| Undetectable HBV DNAa, n (%) | ||
| 12 months | 24 (40.7) | 28 (66.7) |
| 24 monthb | 25 (71.4) | 21 (92.3) |
| ALT normalization, n (%) | ||
| 12 months | 47 (79.7) | 30 (71.4) |
| 24 monthsb | 28 (80) | 14 (60.9) |
| HBeAg loss, n (%) | ||
| 12 months | 11 (18.6) | |
| 24 monthsb | 11 (31.4) | |
| HBeAg seroconversion, n (%) | ||
| 12 months | 9 (15.3) | |
| 24 monthsb | 7 (20) | |
- SD, standard deviation; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transpeptidase; ETV, entecavir; HBV, hepatitis B virus.
- a HBV DNA <50 copies/ml.
- b 35 and 23 in HBeAg(+) and HBeAg(−) patients continued treatment for >24 months, respectively.
Virologic, Serologic, and Biochemical Response to Entecavir
Forty seven (79.7%) and 30 (71.4%) had ALT normalization by 12 months of ETV treatment, in HBeAg-positive and -negative patients, respectively, and 28 of 35 eAg-positive patients (80%) and 14 of 23 HBeAg-negative patients (60.9%) had ALT normalization by 24 months of therapy. ETV-reduced HBV DNA to an undetectable level by PCR (<50 copies/ml) in 24 (40.7%) and 28 (66.7%) patients after 12 months, and 25 (71.4%) and 21 (92.3%) after 24 months of treatment, in HBeAg-positive and -negative patients, respectively. Loss of HBeAg occurred in 11 of 59 (18.6%) and 11 of 35 HBeAg-positive patients (31.4%) by 12 and 24 months of treatment, respectively. HBeAg seroconversion occurred in 9 (15.3%) and seven patients (20%) by 12 and 24 months of treatment, respectively. HBsAg loss was not found during treatment in this population (Table I).
Change of Serum HBsAg Level During ETV Treatment and Correlation With Baseline Factors
After 3 months of treatment, the mean level of serum HBsAg significantly decreased from 3.32 log10 IU/ml at baseline to 3.16 log10 IU/ml (P = 0.035). However, the mean level of serum HBsAg increased to 3.28, 3.32, and 3.35 log10 IU/ml at 6, 12, and 24 months of treatment, respectively. There were no significant differences between the HBsAg level at baseline, and 6, 12, and 24 months of treatment. In HBeAg-positive patients, the mean level of serum HBsAg significantly decreased from 3.51 at baseline to 3.22 log10 IU/ml (P = 0.009) at 3 months. After 3 months, the mean level of serum HBsAg did not show significant change with 3.34, 3.36, and 3.40 log10 IU/ml at 6, 12, and 24 months of treatment, respectively. In HBeAg-negative patients, the mean level of serum HBsAg tended to increase from 3.06 at baseline to 3.09, 3.20, 3.26, and 3.27 log10 IU/ml at 3, 6, 12, and 24 months of treatment, respectively. There were significant changes in HBsAg levels between baseline and 12 months (P = 0.018), 3 and 6 months (P < 0.001), and 6 and 12 months (P = 0.044, Fig. 1).
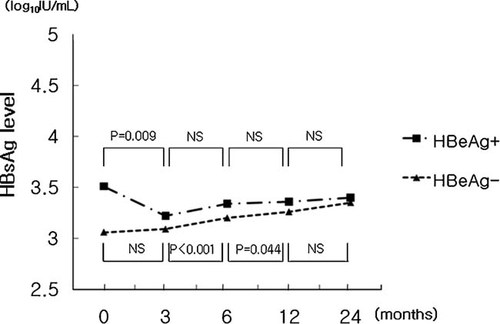
Concentration curves of HBsAg level during entecavir treatment. NS, not significant.
The mean baseline level of serum HBsAg in HBeAg-positive patients was 3.51 log10 IU/ml, which was significantly higher than in HBeAg-negative patients (3.06 log10 IU/ml, P = 0.017). During treatment, the mean HBsAg levels at 3, 6, 12, and 24 months of treatment showed no significant differences between patients with and without HBeAg.
The baseline HBsAg level showed a significant correlation with age (γ = −0.256), AST (γ = −0.309), INR (γ = 0.424), GGT (γ = −0.603), HBV-DNA level (σ = 0.591), HBeAg-positive hepatitis (σ = 0.362), and cirrhosis (σ = −0.271).
Factors Associated With ETV Treatment Responses in HBeAg-Positive Patients
HBV DNA <2,000 copies/ml at 3 months of treatment (P < 0.001), negative HBV PCR at 6 months of treatment (P = 0.002), low levels of mean HBsAg at 3 months of treatment (P = 0.017), and HBsAg level lower than 3,000 IU/ml at 3 months were shown to be associated with undetectable HBV DNA by PCR at 12 months of treatment. Multivariate analysis revealed that HBsAg level lower than 3,000 IU/ml at 3 months [P = 0.001, odds ratio (OR) = 18, 95% confidence interval (CI) = 3.425–94.595] was an independent factor for undetectable HBV DNA at 12 months of treatment.
After 24 months of ETV treatment, HBV-DNA levels <2,000 copies/ml at 3 months of treatment (P = 0.035), negative HBV PCR at 6 (P = 0.034) and 12 months of treatment (P = 0.001), lower level of mean HBsAg at baseline and 3, 6, and 12 months of treatment (P: 0.007, 0.004, 0.006, and <0.001, respectively), and HBsAg level <3,000 IU/ml at 3 months of treatment (P = 0.004) were factors associated with undetectable HBV DNA based on univariate analysis. HBsAg level <3,000 IU/ml at 3 months of treatment was an independent factor for undetectable HBV DNA at 24 months of treatment (P = 0.01, OR = 49, 95% CI = 2.531–948.619, Table II).
| Variables | 12 months | P-value | 24 months | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| PCR (−), n = 24 | PCR (+), n = 35 | Univariate analysis | Multivariate analysis | PCR (−), n = 25 | PCR (+), n = 10 | Univariate analysis | Multivariate analysis | |
| Mean age, years (SD) | 42.67 (±10.15) | 40.43 (±9.19) | 0.382 | 42.84 (±8.81) | 38.80 (±9.53) | 0.239 | ||
| Male, n (%) | 19 (79.2) | 25 (71.4) | 0.558 | 18 (72.0) | 7 (70) | 1.000 | ||
| Mean albumin, mg/dl (SD) | 3.98 (±0.57) | 4.18 (±0.41) | 0.141 | 4.00 (±0.53) | 4.18 (±0.35) | 0.326 | ||
| Mean platelet, 103/mm3 (SD) | 169.26 (±52.2) | 180.29 (±54.21) | 0.491 | 156.26 (±47.44) | 186.50 (±44.38) | 0.136 | ||
| Mean AST, IU/L (SD) | 215.96 (±296.80) | 104.77 (±65.20) | 0.083 | 197.08 (±275.14) | 104.00 (±41.14) | 0.299 | ||
| Mean ALT, IU/L (SD) | 294.08 (±399.17) | 182.51 (±123.95) | 0.196 | 281.36 (±399.51) | 231.60 (±154.48) | 0.707 | ||
| Mean TB, mg/dl (SD) | 1.50 (±1.26) | 1.13 (±1.03) | 0.227 | 1.33 (±1.12) | 0.97 (±0.59) | 0.346 | ||
| Liver cirrhosis, n (%) | 4 (16.7) | 7 (20.0) | 1.000 | 7 (28) | 1 (10) | 0.390 | ||
| HBV DNA, log10 copies/ml, n (%) | ||||||||
| ≤ 8 | 10 (41.7) | 11 (31.4) | 0.581 | 10 (40) | 3 (30) | 0.709 | ||
| HBV DNA PCR (−), n (%) | ||||||||
| 3 monthsa | 12 (52.2) | 2 (6.3) | <0.001 | 0.001 | 9 (39.1) | 0 (0) | 0.035 | 0.686 |
| 6 months | 12 (50.0) | 4 (11.4) | 0.002 | 0.092 | 10 (40) | 0 (0) | 0.034 | 0.408 |
| 12 months | 16 (64) | 0 (0) | 0.001 | 0.998 | ||||
| Mean HBsAg, log10 IU/ml (SD) | ||||||||
| Baseline | 3.26 (±1.11) | 3.68 (±1.01) | 0.140 | 3.23 (±1.11) | 4.33 (±0.76) | 0.007 | 0.218 | |
| 3 months | 2.83 (±1.07) | 3.49 (±0.89) | 0.017 | 0.423 | 2.82 (±1.09) | 4.01 (±0.40) | 0.004 | 0.982 |
| 6 months | 3.06 (±0.97) | 3.52 (±0.78) | 0.057 | 2.97 (±1.00) | 3.98 (±0.38) | 0.006 | 0.253 | |
| 12 months | 3.04 (±0.82) | 3.87 (±0.25) | <0.001 | 0.219 | ||||
| HBsAg <3,000 IU/ml at 3 months, n (%) | 17 (77.3) | 14 (43.8) | 0.024 | 0.173 | 17 (73.9) | 1 (11.1) | 0.004 | 0.010 |
| Decline >1 log10 IU/ml, n(%) | ||||||||
| 3 months | 5 (22.7) | 3 (9.4) | 0.248 | 4 (17.4) | 1 (11.1) | 1.000 | ||
| 6 months | 3 (13) | 2 (5.9) | 0.384 | 3 (12.5) | 1 (11.1) | 1.000 | ||
- AST, aspartate aminotransferase; ALT, alanine aminotransferase; TB, total bilirubin.
- a Decline of HBV DNA <2,000 copies/ml using b-DNA assay after 3 months of therapy.
Univariate analysis revealed that AST level (P = 0.042), HBV-DNA levels <2,000 copies/ml at 3 months of treatment (P = 0.010), negative HBV PCR at 6 months of treatment (P = 0.035), low level of HBsAg at baseline and 3 and 6 months of treatment (P: 0.004, 0.007, and 0.009, respectively), HBsAg level <3,000 IU/ml at 3 months of treatment (P = 0.004), and decline in HBsAg >1 log10 IU/ml at 6 months of treatment (P = 0.047) were factors associated with HBeAg loss or seroconversion at 12 months of treatment. In stepwise regression modeling, HBV-DNA levels <2,000 copies/ml at 3 months of treatment (P = 0.046, OR = 4.432, 95% CI = 1.025∼19.158) and HBsAg level <3,000 IU/ml at 3 months of treatment (P = 0.026, OR = 5.339, 95% CI = 1.227–23.221) were independent predictive factors of ETV induced HBeAg loss or seroconversion.
After 24 months of treatment, the followings were significantly associated with HBeAg loss or seroconversion based on univariate analysis: low hemoglobin level (P = 0.020); and low level of serum HBsAg at baseline and 3, 6, and 12 months (P = 0.001, 0.018, 0.006, and 0.021, respectively). The level of serum HBsAg at baseline was an independent predictive factor of ETV-induced HBeAg loss or seroconversion (P = 0.046, OR = 0.178, 95% CI = 0.033∼0.097). However, the HBV-DNA level at baseline and during treatment did not show significant differences between patients with and without HBeAg loss or seroconversion at 24 months (Table III).
| Variables | 12 months | P-value | 24 months | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Yes, n = 20 | No, n = 39 | Univariate analysis | Multivariate analysis | Yes, n = 18 | No, n = 17 | Univariate analysis | Multivariate analysis | |
| Mean age, years (SD) | 43.90 (±10.37) | 40.03 (±9.00) | 0.142 | 44.39 (±9.53) | 38.82 (±7.83) | 0.069 | ||
| Male, n (%) | 12 (60) | 32 (82.1) | 0.112 | 10 (55.6) | 15 (88.2) | 0.060 | ||
| Mean albumin, mg/dl (SD) | 4.00 (±0.66) | 4.15 (±0.36) | 0.352 | 3.92 (±0.56) | 4.21 (±0.34) | 0.093 | ||
| Mean Hb, mg/dl (SD) | 13.79 (±1.53) | 14.61 (±1.69) | 0.128 | 13.89 (±1.48) | 15.28 (±1.43) | 0.020 | 0.456 | |
| Mean platelet, 103/mm3 (SD) | 156.71 (±55.53) | 183.94 (±50.78) | 0.109 | 150.79 (±51.46) | 180.77 (±39.75) | 0.104 | ||
| Mean AST, IU/L (SD) | 253.55 (±319.39) | 96.90 (±47.02) | 0.042 | 0.083 | 228.72 (±317.50) | 108.82 (±58.36) | 0.133 | |
| Mean ALT, IU/L (SD) | 313.75 (±418.80) | 183.87 (±145.90) | 0.192 | 295.00 (±451.72) | 237.65 (±188.00) | 0.631 | ||
| Mean TB, mg/dl (SD) | 1.71 (±1.34) | 1.06 (±0.95) | 0.068 | 1.47 (±1.29) | 0.97 (±0.48) | 0.141 | ||
| Liver cirrhosis, n (%) | 5 (25) | 6 (15.4) | 0.483 | 6 (33.3) | 2 (11.8) | 0.228 | ||
| HBV DNA, log10 copies/ml, n(%) | ||||||||
| ≤8 | 10 (50) | 11 (28.2) | 0.151 | 8 (44.4) | 5 (29.4) | 0.489 | ||
| HBV DNA PCR (−), n (%) | ||||||||
| 3 monthsa | 9 (47.4) | 5 (13.9) | 0.010 | 0.046 | 7 (43.8) | 2 (12.5) | 0.113 | |
| 6 months | 9 (45) | 7 (17.9) | 0.035 | 0.884 | 7 (38.9) | 3 (17.6) | 0.264 | |
| 12 months | 11 (61.1) | 5 (29.4) | 0.092 | |||||
| Mean HBsAg, log10 IU/ml (SD) | ||||||||
| Baseline | 2.98 (±1.26) | 3.79 (±0.83) | 0.004 | 0.629 | 2.98 (±1.19) | 4.14 (±0.67) | 0.001 | 0.046 |
| 3 months | 2.72 (±1.21) | 3.49 (±0.79) | 0.007 | 0.601 | 2.71 (±1.27) | 3.60 (±0.65) | 0.018 | 0.239 |
| 6 months | 2.85 (±1.10) | 3.60 (±0.60) | 0.009 | 0.550 | 2.85 (±1.12) | 3.73 (±0.47) | 0.006 | 0.239 |
| 12 months | 2.98 (±0.93) | 3.59 (±0.49) | 0.021 | 0.438 | ||||
| HBsAg <3,000 IU/ml at 3 months, n (%) | 16 (84.2) | 15(42.9) | 0.004 | 0.026 | 12 (75) | 6 (37.5) | 0.073 | |
| Decline >1 log10 IU/ml, n(%) | ||||||||
| 3 months | 5 (26.3) | 3 (8.6) | 0.113 | 3 (18.8) | 2 (12.5) | 1.000 | ||
| 6 months | 4 (20) | 1 (2.7) | 0.047 | 0.222 | 3 (16.7) | 1 (6.7) | 0.607 | |
- Hb, hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TB, total bilirubin.
- a Decline of HBV DNA <2,000 copies/ml using b-DNA assay after 3 months of therapy.
Factors Associated With ETV Treatment Responses in HBeAg-Negative Patients
Liver cirrhosis (P = 0.049) and negative HBV PCR at 6 months of treatment (P = 0.003) were shown to be associated with undetectable HBV DNA by PCR at 12 months of treatment. Negative HBV PCR at 6 months of treatment was an independent factor for undetectable HBV DNA at 12 months of treatment (P = 0.008, OR = 11.115, 95% CI = 1.892∼65.310, Table IV). After 24 months of treatment, 21 of 23 patients (92.3%) had negative HBV PCR, and there was no significant predicting factor associated with undetectable HBV DNA.
| Variables | PCR (−), n = 28 | PCR (+), n = 14 | P-value | |
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| Mean age, years (SD) | 45.68 (±8.66) | 48.57 (±8.09) | 0.304 | |
| Male, n (%) | 20 (71.4) | 10 (71.4) | 1.000 | |
| Mean albumin, mg/dl (SD) | 4.09 (±0.45) | 4.01 (±0.45) | 0.353 | |
| Mean platelet, 103/mm3 (SD) | 141.68 (±47.66) | 137.57 (±64.63) | 0.853 | |
| Mean AST, IU/L (SD) | 114.39 (±85.20) | 104.43 (±85.08) | 0.723 | |
| Mean ALT, IU/L (SD) | 182.18 (±140.80) | 125.29 (±57.97) | 0.156 | |
| Mean TB, mg/dl (SD) | 1.34 (±1.41) | 1.17 (±0.45) | 0.662 | |
| Liver cirrhosis, n (%) | 10 (35.7) | 10 (71.4) | 0.049 | 0.055 |
| HBV DNA, log10 copies/ml | 0.491 | |||
| >8 | 7 (25) | 5 (35.7) | ||
| ≤8 | 21 (75) | 9 (64.3) | ||
| HBV DNA PCR (−), n (%) | ||||
| 3 monthsa | 22 (78.6) | 8 (57.1) | 0.169 | |
| 6 months | 18 (64.3) | 2 (14.3) | 0.003 | 0.008 |
| Mean HBsAg, log10 IU/ml | ||||
| Baseline | 2.98 (±0.79) | 3.22 (±0.42) | 0.292 | |
| 3 months | 3.05 (±0.53) | 3.16 (±0.35) | 0.494 | |
| 6 months | 3.18 (±0.56) | 3.24 (±0.34) | 0.707 | |
| HBsAg <3,000 IU/ml, n (%) | ||||
| 3 months | 19 (70.4) | 11 (78.6) | 0.719 | |
| Decline >1 log10 IU/ml, n (%) | ||||
| 3 months | 3 (11.1) | 2 (14.3) | 1.000 | |
| 6 months | 2 (7.4) | 1 (7.1) | 1.000 | |
- AST, aspartate aminotransferase; ALT, alanine aminotransferase; TB, total bilirubin.
- a Decline of HBV DNA <2,000 copies/ml using b-DNA assay after 3 months of therapy.
DISSCUSSION
The mean HBsAg level significantly decreased by 3 months of ETV treatment, then increased by 6, 12, and 24 months of treatment, resulting in no significant differences compared to the HBsAg level at baseline. It has been reported that the serum HBsAg level was well-correlated with the intrahepatic cccDNA level [Wursthorn et al., 2006; Ozaras et al., 2008]. The initial decline in serum HBsAg may be due to the potent suppression of viral DNA synthesis, which would effectively deplete the pool of mature cytoplasmic nucleocapsids available for conversion to cccDNA. However, the HBsAg level did not change significantly compared with the baseline level after 6 months of treatment. It is thought that ETV, like other nucleoside analogs, is limited in suppressing remnant intrahepatic cccDNA because ETV mainly acts on suppression of viral replication within the cytoplasm.
The pattern of changes in the HBsAg level during treatment was shown to be different between patients with and without HBeAg. At 3 months of ETV treatment, a significant decline in the HBsAg level occurred in HBeAg-positive patients. However, in HBeAg-negative patients, there was no initial decline and a significant increase after 12 months of treatment (P = 0.018). HBsAg levels have recently been reported to have positive correlation with serum HBV DNA and intrahepatic cccDNA in HBeAg-positive patients, but not in HBeAg-negative patients [Thompson et al., 2010]. The cause of different pattern in the HBsAg level between these two groups needs to be validated in further studies.
In a recent study involving 28 HBeAg-positive patients, a significant decrease in the HBsAg concentration from the baseline level during treatment with ETV was reported [Jung et al., 2010]. However, in the study, the number of enrolled patients was small and there was no significant change in the HBsAg level between 6 and 12 months of treatment, although a significant decline was observed during the initial 6 months of treatment. The finding of an early decline in the HBsAg level during ETV treatment is consistent with the findings in the current study.
The HBsAg level showed a strong positive correlation with the HBV-DNA level (P < 0.001, σ = 0.591). Brunetto et al. [2009] reported similar data showing a positive correlation between serum HBsAg and serum HBV-DNA levels before pegylated interferon treatment in HBeAg-negative patients, but there was no apparent correlation with the ALT level or age.
The independent factor associated with an undetectable HBV-DNA level by PCR after 12 months of ETV treatment was a decline in the HBV-DNA level to <2,000 copies/ml in HBeAg-positive patients. A HBV-DNA level <2,000 /ml at 3 months of treatment was also an independent factor predicting HBeAg loss or seroconversion after 12 months of ETV treatment, along with a serum HBsAg level <3,000 IU/ml at 3 months of treatment. Treatment of CHB patients with potent nucleos(t)ides, such as ETV or adefovir, can result in partial restoration of immune responses, which are necessary for the durable host-mediated control of infection. It is thought that the initial response to the drug may reflect this restoration of immune responses in the host [Cooksley et al., 2002; Werle-Lapostolle et al., 2004].
In HBeAg-positive patients, the serum HBsAg level was a useful predictor of the ETV response after 24 months of therapy, as shown in other studies of peginterferon treatment in patients with CHB [Buster et al., 2008; Lau et al., 2008; Brunetto et al., 2009; Moucari et al., 2009; Tangkijvanich et al., 2009]. Jung et al. [2010] reported that patients with a sufficient HBsAg drop had greater HBeAg loss by 1 year of treatment with ETV. In the current study, a serum HBsAg level <3,000 IU/ml at 3 months was an only independent predicting factor associated with undetectable DNA PCR, and the low levels of serum HBsAg at baseline was an independent factor associated with HBeAg loss or seroconversion at 24 months of therapy.
Low levels of serum HBsAg by baseline and 3, 6, and 12 months of treatment were also associated with HBeAg loss or seroconversion after 24 months of ETV treatment, along with low level of hemoglobin based on univariate analysis. The negative correlation between HBsAg and age was shown in this study. Fan et al. [2001] reported that an age-related decrease in serum HBsAg levels may be due to low viral replication related to emergence of the pre-S deletion mutants. Also, the level of hemoglobin was significantly correlated with age (P = 0.032, Pearson correlation = −0.241), in the current study. Therefore, hemoglobin level may be correlated with the HBsAg level.
As previous other studies, after 12 months of ETV therapy, early decrease of HBV-DNA level was a significant predicting factor of treatment response in the current study. However, after 24 months of ETV therapy, the lower level of HBsAg was a significant predicting factor of undetectable HBV DNA and HBeAg loss/seroconversion. In HBeAg-postive patients, the levels of serum HBV DNA at baseline and during treatment did not show significant differences between patients with and without HBeAg loss or seroconversion at 24 months of treatment. Therefore, the level of serum HBsAg may be a better predictor of long-term treatment response than the serum HBV-DNA level in HBeAg-positive patients receiving ETV. It has been reported that a HBsAg level <2 log10 IU/ml at treatment week 104 was highly predictive of a sustained virologic response at 2 years off-treatment in patients treated with telbivudine [Cai et al., 2010]. A prospective, long-term study with a larger number of patients is needed to determine the correlation between the level of HBsAg and the ETV off-treatment response.
In HBeAg-negative patients, undetectable HBV DNA at 6 months was an independent factor predicting undetectable HBV DNA after 12 months of ETV therapy. HBsAg levels at baseline and during treatment did not show any correlation with the ETV response. After 24 months of therapy, majority of patients (92.3%) had undetectable HBV DNA, and there was no significant factor associated with the ETV response. ETV is a potent antiviral drug and negative HBeAg, itself is a strong predicting factor associated with a good treatment response. Therefore, with long-term ETV treatment alone, satisfactory responses could be expected in HBeAg-negative patients.
The analysis of genotype was not performed, because HBV infection in Korea is almost exclusively genotype C [Lee et al., 2004]. The results of this study could be applicable to CHB patients with genotype C.
In conclusion, the level of serum HBsAg did not show a significant change compared with the baseline level until 24 months of ETV treatment in naïve CHB patients, although there was a significant decline in the early stage of treatment. The pattern of change in the HBsAg level during treatment was different between HBeAg-positive and HBeAg-negative patients. The level of serum HBsAg was a good predictor of undetectable HBV DNA and HBeAg loss/seroconversion in HBeAg-positive patients. However, in HBeAg-negative patients, HBsAg level did not show any difference between with and without treatment response. Measurement of the serum HBsAg level may be useful for predicting the long-term response of ETV in treatment-naïve HBeAg-positive patients.
Acknowledgements
This study was supported in part by a generous donation of samples from the Biobank of Ajou University hospital (A Biobank in the KOBRIN network).




