Human T cell lymphotropic virus type 1 (HTLV-1) proviral load of HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients according to new diagnostic criteria of HAM/TSP†
M Fernanda, R Grassi, and VN Olavarria contributed equally to this work.
Abstract
A high human T-cell lymphotropic virus type 1 (HTLV-1) proviral load is described in HTLV-1-associated diseases, especially HAM/TSP. However, the cut-off value to define high levels of HTLV-1 proviral load is not well established. 281 HTLV-1-infected patients from the HTLV reference center in Salvador, Brazil, were followed from 2005 to 2008. Patients were classified as asymptomatic, possible-, probable-, and definite-HAM/TSP, in accordance with diagnostic criteria proposed by De Castro-Costa et al. (2006): AIDS Res Hum Retroviruses 22:931–935. HTLV-1 proviral load was determined using real-time PCR. A receiver operator characteristic (ROC) curve was constructed using only asymptomatic individuals and definite-HAM/TSP patients. The ROC curve was used to predict the proviral load level that differentiates these two groups. Out of 281 patients, 189 were asymptomatic and 92 were diagnosed with HAM/TSP (22 possible, 23 probable, 47 definite). The mean HTLV-1 proviral load was higher in possible- (89,104 ± 93,006 copies/106 PBMC), -probable (175,854 ± 128,083 copies/106 PBMC), and definite-HAM/TSP patients (150,667 ± 122,320 copies/106 PBMC), when compared to asymptomatic individuals (27,178 ± 41,155 copies/106 PBMC) (P < 0.0001). A comparison of all HAM/TSP groups showed the highest proviral loads in probable-HAM/TSP patients, yet the differences in mean values were not statistically significant. The ROC curve suggested a value of 49,865 copies/106 PBMC, with 87% sensitivity (95% CI = 74–95) and 81% specificity (95% CI = 75–86), as the best proviral load cut-off point to differentiate definite HAM/TSP patients from asymptomatic individuals. HTLV-1 proviral loads are higher in groups of infected patients with neurological symptoms and may represent a relevant biological marker of disease progression. J. Med. Virol. 83:1269–1274, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Human T-cell lymphotropic virus type 1 (HTLV-1) is a human retrovirus that infects roughly 10–20 million people worldwide [de The and Bomford, 1993]. However, HTLV-1 infection rates vary according to population subgroups and geographic location studied [Hlela et al., 2009]. Large foci of HTLV-1 are found in southern Japan, the Caribbean, Central and West Africa, and South America [Proietti et al., 2005]. In Brazil, the highest prevalence of HTLV-1 was estimated in the general population of Salvador (1.7%), a city with three million inhabitants located in the northeastern region of the country [Dourado et al., 2003].
HTLV-1 is the etiological agent of HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP), adult T-cell leukemia (ATL) and HTLV-1-associated uveitis (HAU) [Hinuma et al., 1981; Gessain et al., 1985; Osame et al., 1986; Mochizuki et al., 1992]. It has been reported that less than 5% of infected individuals will develop an HTLV-1-associated disease. However, there is evidence that HTLV-1 is associated with a broader spectrum of other diseases, including arthritis [Ijichi et al., 1990], polymyositis [Morgan et al., 1989], and lymphocytic interstitial pneumonia [Setoguchi et al., 1991], as well as leading to a higher prevalence of other infectious diseases, such as tuberculosis [Marinho et al., 2005; Verdonck et al., 2007], strongyloidiasis [Nakada et al., 1984; Gotuzzo et al., 2007], scabies [Brites et al., 2002; Blas et al., 2005], and infective dermatitis in children [LaGrenade et al., 1990].
HAM/TSP is a debilitating neurological disorder causing spastic paraparesis, which leads to a progressive loss of motor function in the lower limbs. Additionally, bladder and bowel dysfunction, as well as superficial and deep sensory disturbances in lower extremities, including paresthesia and dysesthesia, are reported by patients. The diagnosis of HAM/TSP is based on an extensive list of both clinical and laboratory criteria, as proposed by the World Health Organization in 1988 and revised in 1989. Recently, De Castro-Costa et al. [2006] proposed simplified HAM/TSP diagnostic criteria, consisting of three levels of ascertainment: possible, probable, and definite [De Castro-Costa et al., 2006]. The advantage of these proposed criteria is disease classification at an earlier stage, when patients may present only isolated symptoms.
HTLV-1 predominantly infects activated memory CD4 T-cells [Richardson et al., 1990]. The HTLV-1 provirus remains integrated into the host's genome over a lifetime and the HTLV-1 proviral load (amount of integrated virus) is mainly sustained through the mitotic division of infected cells [Asquith and Bangham, 2007]. However, each host's proviral load remains stable over time, probably because the specific HTLV-1 cytotoxic response of CD8 T-cells eliminates a portion of the infected cells [Asquith and Bangham, 2007].
Several studies have described an association between a higher HTLV-1 proviral load in HAM/TSP patients when compared to asymptomatic individuals [Nagai et al., 1998; Olindo et al., 2005]. However, the cut-off value for defining a high level of HTLV-1 proviral load has not been well-defined. The aim of this study was to establish a high proviral load cut-off value to differentiate asymptomatic individuals from HAM/TSP patients, in accordance with the De Castro-Costa diagnostic criteria.
METHODS
All patients included in this study were followed at the Bahia School of Medicine and Public Health HTLV reference center in Salvador, located in northeastern Brazil, from 2005 to 2008. This is a free public outpatient clinic that has provided comprehensive care to a total of 1,050 patients since 2002. Of these, 50% are regularly seen at least twice a year. A total of 281 patients were included in the present study with inclusion criteria consisting of an available HTLV-1 proviral load measurement and a neurological evaluation. Myelopathic symptoms, serological findings, and/or detection of HTLV-1 DNA, as well as the exclusion of other disorders were used as indicators for the diagnostic categorization of HAM/TSP according to ascertainment level (possible, probable, and definite) [De Castro-Costa et al., 2006]. Co-infected patients with HIV and/or HCV were excluded, as well as symptomatic patients with other HTLV-1-associated diseases, such as infective dermatitis, uveitis, ATL, Sicca syndrome, etc. The Disability Status Scale (DSS) [Kurtzke, 1955] and the Osame Motor Disability Score (OMDS) [Izumo et al., 1996] were regularly applied by a neurologist. DSS was quantified from 0 (normal) to 10 (death). OMDS was graded from 0 (normal walking and running) to 13 (completely bedridden). The diagnosis of HTLV-1 infection was performed using ELISA (Cambridge Biotech Corp., Worcester, MA) and was confirmed by Western Blot analysis (HTLV blot 2.4, Genelab, Singapore). This study was approved by the Institutional Review Board of the Oswaldo Cruz Foundation, Brazilian Ministry of Health. Informed consent was obtained from all enrolled patients.
Measurement of HTLV-1 Proviral Load
PBMCs were obtained from EDTA blood by density gradient centrifugation and cryopreserved until use. All experiments were performed at LASP-FIOCRUZ-Bahia. DNA was extracted using spin column DNA extraction system (Qiagen, Hilden, Germany). HTLV-1 proviral load was quantified using a real-time TaqMan polymerase chain reaction (PCR) method, as described previously [Dehee et al., 2002]. Briefly, SK110/SK111 primers were used to amplify a 186 bp fragment of the pol gene and dual TaqMan probe (5′-FAM/5′ VIC and 3′-TAMRA) was located at 4,829–4,858 bp of the HTLV-1 reference sequence (HTLVATK). Albumin DNA was used as an endogenous reference. The value of HTLV-1 proviral load was reported as the [(HTLV-1 average copy number)/(albumin average copy number)] × 2 × 106 and expressed as the number of HTLV-1 copies per 106 cells in PBMCs.
Analysis
Median values of HTLV-1 proviral loads, time of symptoms duration (years) and grade of neurological and motor disability (DSS and OMDS) were calculated for all patient groups. Kruskal–Wallis non-parametric analysis of variance with the Bonferroni-Dunn multiple comparison tests was used to compare asymptomatic and possible-, probable-, and definite-HAM/TSP groups. The Fisher exact chi-square test was used to compare gender proportions. The receiver operator characteristic (ROC) curve and the area under the curve were used in order to identify the HTLV-1 proviral load level that differentiates asymptomatic individuals from definite-HAM/TSP patients. The cut-off point was determined by the value that exhibited an equilibrium between the highest levels of sensitivity and specificity (point of equilibrium). The level of statistical significance for the P-value was set at <0.05. Graphpad Prism (5.0; La Jolla, CA) was used for statistical calculations.
RESULTS
Out of a total of 281 HTLV-1-infected individuals, 189 were asymptomatic and 92 had HAM/TSP. Of these, 22 were classified as possible-HAM/TSP, 23 as probable-, and 47 as definite-. The proportion of females was similar in all groups. The median age of the possible- and definite-HAM/TSP groups was significantly higher, when compared to asymptomatic individuals. In addition, the median age of the possible-HAM/TSP group was significantly higher than the probable-HAM/TSP group (Table I).
| Asymptomatic (n = 189) | HAM/TSP | P-value | |||
|---|---|---|---|---|---|
| Possible (n = 22) | Probable (n = 23) | Definite (n = 47) | |||
| Female, n (%) | 132 (70) | 16 (76) | 15 (62) | 35 (73) | 0.74* |
| Age, median (range) | 41 (10–82) | 58 (36–73) | 42 (27–66) | 55 (30–79) | <0.001** |
- * Fisher exact chi-square test.
- ** Kruskal–Wallis test with the Bonferroni-Dunn multiple comparisons. The level of significance was set at P < 0·05. Significant differences occurred between asymptomatic and possible-HAM/TSP groups, asymptomatic and definite-HAM/TSP groups and in possible- and definite-HAM/TSP groups.
All HAM/TSP groups (possible, probable, and definite) had median HTLV-1 proviral loads significantly higher than those of asymptomatic individuals (Table II and Fig. 1). The median HTLV-1 proviral load of probable-HAM/TSP patients (147,641 copies/106 PBMC) was higher than those of possible (45,972 copies/106 PBMC) and definite-HAM/TSP patients (116,424 copies/106 PBMC); however, this difference was not statistically significant. Duration of neurological symptoms was similar in all HAM-TSP groups, while the grade of neurological and motor dysfunction measured by OMDS and DSS grade progressively increases from possible- to definite-HAM/TSP patients (Table II).
| Asymptomatic (n = 189) | HAM/TSP | P-value* | |||
|---|---|---|---|---|---|
| Possible (n = 22) | Probable (n = 23) | Definite (n = 47) | |||
| Proviral load | |||||
| Median (IQR) | 7,109 (568–36,319) | 45,972 (32,607–154,903) | 147,641 (79,602–257,129) | 116,424 (83,591–203,175) | <0.001 |
| Mean ± SD | 27,178 ± 41,155 | 89,104 ± 93,006 | 175,854 ± 128,083 | 150,667 ± 122,320 | |
| Symptoms duration (years) | 6 (0.5–20) | 5 (2–20) | 9 (2–21) | 0.21 | |
| OMDS | 0 | 2 (0–3) | 3 (1–7) | 4 (0–10) | 0.048 |
| DSS | 0 | 3 (0–4) | 4 (1–6) | 6 (0–7) | 0.006 |
- IQR, interquartile range; SD, standard deviation; OMDS, Osame Motor Disability Score; DSS, Disability Status Scale.
- HTLV-1 proviral load (copies/106 PBMC).
- * Data of years of symptoms duration, OMDS and DSS are expressed as median and ranges asymptomatic patients were excluded in the analyses of OMDS and DSS, Kruskal–Wallis P < 0.05.
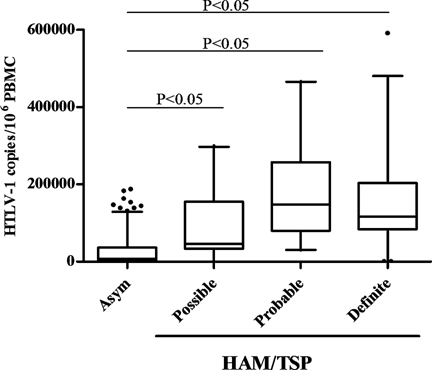
Median of HTLV-1 proviral load in HTLV-1 infected individuals, ranging from asymptomatic individuals (n = 189) and possible-HAM/TSP (n = 22), probable-HAM/TSP (n = 23) and definite-HAM/TSP patients (n = 47). Results are presented as number of HTLV-1 copies/106 PBMC. The box-plots represent the median, interquartile range (boxes) and the 5–95% data range (whisker caps). Circles represent outlier values. The Kruskal–Wallis non-parametric analysis of variance with the Bonferroni-Dunn post-test was used to identify statistically significant differences in proviral load among groups. At a significance level of P < 0.05, statistically significant differences were found only between asymptomatic and possible-, asymptomatic- and probable-, and asymptomatic and definite-HAM/TSP groups.
The ROC curve demonstrated that HTLV-1 proviral load significantly discriminates asymptomatic from definite-HAM/TSP patients, with an area under the ROC curve of 0.88 (95%CI = 0.82–0.94, P < 0.0001). The ROC curve suggests a value of 49,865 copies/106 PBMC as the best cut-off point of HTLV-1 proviral load as a biomarker for clinical monitoring of HAM/TSP diagnosis, with 87% sensitivity (95%CI = 74–95) and 81% specificity (95% CI = 75–86) (Fig. 2). The positive and negative predictive values for the cut-off HTLV-1 proviral load level were 53% (95% CI = 42% a 64%) and 96% (95% CI = 92% a 98%), respectively.
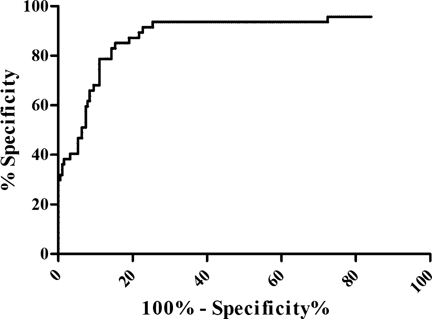
ROCs curve of HTLV-1 proviral load as predictors of HAM/TSP diagnosis. 189 HTLV-1-infected asymptomatic individuals and 47 definite-HAM/TSP patients, area under curve 0.88 (95%CI = 0.82–0.95), P < 0.0001.
The proportion of HTLV-1-infected patients with a proviral load above 49,865 copies/106 PBMC progressively increases in accordance with the degree of diagnostic ascertainment: possible- (45%), probable- (73%), and definite- (85%) HAM/TSP groups. The proportion of asymptomatic individuals with a proviral load above the cut-off value was 19% (Table III).
| Proviral load cut-off value | Asymptomatic N = 189 | HAM/TSP | ||
|---|---|---|---|---|
| Possible-N = 22 | Probable-N = 23 | Definite-N = 47 | ||
| <49,865 | 154 (81) | 12 (55) | 4 (17) | 7 (15) |
| ≥49,865 | 35 (19) | 10 (45) | 19 (73) | 40 (85) |
- Proviral load (copies/106 PBMC), data represent n (%).
DISCUSSION
This study demonstrated for the first time that the level of HTLV-1 proviral load progressively increases according to HAM/TSP ascertainment level (possible, probable, and definite) of De Castro-Costa diagnostic criteria. Moreover, the ROC curve suggested a cut-off value of 49,865 HTLV copies/106 PBMC as the best level of HTLV-1 proviral load to discriminate asymptomatic from definite-HAM/TSP groups. The clinical HAM/TSP diagnostic criteria proposed by De Castro-Costa et al. [2006] attempts to simplify WHO diagnostic criteria for HAM/TSP [Osame et al., 1986; World Health Organization Scientific Group, 1989], which has not been widely employed in HTLV-1 studies. This new proposed criteria includes the most important aspects outlined in the WHO diagnostic guidelines and permits the diagnosis and follow-up of HTLV-1-infected individuals with few myelopathic symptoms. In the possible-HAM/TSP group, disorders that may resemble HAM/TSP are not excluded. In addition, these patients may present a broad spectrum of clinical manifestations. In this group, almost 50% of patients had proviral loads above the cut-off value predicted by the ROC curve. In both probable- and definite-HAM/TSP groups, only 22% and 17% of proviral loads were below this value, respectively. In the probable-HAM/TSP group, in contrast to the possible-HAM/TSP group, other disorders that may resemble HAM/TSP have already been excluded. However, these patients do not meet WHO criteria for a definite-HAM/TSP diagnosis. Since probable- and definite-HAM/TSP groups had similar proviral loads, a reclassification of patients should be considered. If proviral load were to be included as an additional criterion to diagnose HAM/TSP, 45% and 73% of patients from possible- and probable-groups, respectively, would be reclassified in the definite-HAM/TSP group.
In almost 20% of asymptomatic individuals, a proviral load above the established cut-off level was observed; however, it is not known whether these asymptomatic individuals will develop HAM/TSP disease. This phenomenon may be representative of inter-patient variability in HTLV proviral loads at a single time point, since the level of proviral load varies widely between carriers, yet remains relatively constant within an individual over time [Kwaan et al., 2006]. The progression to HAM/TSP is multifactorial and is not only dependent on the virus, but also of the host's genetic background. The HLA-A*02 and Cw*08 alleles have been associated with a lower proviral load and significantly reduced HAM/TSP development, while HLA-DR1 and HLA–B*5401 have been associated with HAM/TSP disease development [Jeffery et al., 1999; Jeffery et al., 2000].
Several studies indicate that a high HTLV-1 proviral load is associated with HAM/TSP, since the proviral load in HAM/TSP patients is higher than that of asymptomatic individuals [Nagai et al., 1998; Olindo et al., 2005; Silva et al., 2007]. In addition, patients with isolated neurological abnormalities, such as mild cognitive deficits, peripheral neuropathy, or neurogenic bladder dysfunction, present higher HTLV-1 proviral loads compared to asymptomatic individuals [Silva et al., 2007]. An increased HTLV-1 proviral load is also present in patients with other HTLV-1-associated diseases, such as infective dermatitis [Primo et al., 2009], ATL [Okayama et al., 2004], as well as in HTLV-1-infected patients with rheumatoid arthritis or other connective tissue diseases [Yakova et al., 2005]. However, due to methodological differences in measuring HTLV-1 proviral load, the proportion of HTLV-1 infected cells varied among studies, making the establishment of a high proviral load cut-off value difficult. A proviral load has been considered to be low if the proportion of infected PBMC is <1%, intermediate if between 1 and 5%, and high if >5% [Goncalves et al., 2008]. In the present study, possible-HAM/TSP patients showed a median proviral load of 46,153 copies/106 PBMC, while probable- and definite-HAM/TSP patients presented levels 19 and 16 times higher, respectively, when compared to asymptomatic individuals. The authors propose that levels of proviral load higher than 50,000 HTLV-1 copies/106 PBMC should be considered as an additional criterion to diagnosis HAM/TSP disease in the patients of the probable-group.
This study is limited by its cross-sectional design and the fact that the implications of a high HTLV-1 proviral load on asymptomatic individuals have not been addressed. Furthermore, since there is no golden standard for the diagnosis of HAM/TSP, the subdivision of this diagnosis into possible-, probable-, and definite- categories is solely based on clinical criteria. Therefore, it is not possible to assess the true predictive value of proviral load in patients with a possible- or probable-HAM/TSP diagnosis. Lastly, the incidence of HAM/TSP in HTLV-1-infected individuals is very low (<2%) while the disease is insidious. Our group is currently following a large cohort of HTLV-1-infected patients in order to evaluate the development of HAM/TSP in these individuals.
In summary, the results presented herein confirm that HTLV-1 proviral load is higher in HAM/TSP patients than asymptomatic individuals, regardless of diagnostic ascertainment level. Thus, proviral load should be considered as a possible additional criterion in the diagnosis of HAM/TSP in patients with myelopathic symptoms. Additional longitudinal studies should be carried out to further evaluate this possibility.
Acknowledgements
This study was supported by the Ministério da Saúde Programa Nacional de DST/aids and the Fundação de Amparo a Pesquisa da Bahia (FAPESB). We thank Dr. Raymond Césarie for providing HTLV/Albumina clones and Andris K. Walter for his assistance in English revision.




