Exposure to multiple subgenotypes of hepatitis a virus during an outbreak using matched serum and saliva specimens
Abstract
Matched serum and saliva samples were collected simultaneously from 124 subjects exposed during a hepatitis A virus (HAV) outbreak at a daycare center in Rio de Janeiro, Brazil. All samples were tested for IgM and total anti-HAV antibodies by enzyme immunoassay (EIA). HAV was detected by nested PCR in serum, saliva, and water samples employing primers for the VP1/2A region of the viral RNA; all positive products were then sequenced. The viral load of the matched samples was determined by real-time PCR using the TaqMan system. HAV-RNA was identified by nested PCR in 37.7% of the saliva samples, 29% of the serum samples, and one drinking water sample. The mean HAV viral load was similar in the serum and saliva specimens (103 copies/ml). HAV genotypes IA and IB were detected in both specimen types, and the water sample isolate was classified as genotype IB, indicating the existence of more than one source of infection at the daycare center. In six infected patients, a different HAV subgenotype was found in their serum than in their saliva, and this unusual pattern of mixed HAV infection was investigated further by molecular cloning followed by nucleotide sequencing. All clones derived from the saliva samples belonged to subgenotype IB and shared 96.5–100% identity. However, clones derived from their corresponding serum sample belonged to subgenotype IA and shared 90.5–100% identity. This study showed the important role that non-invasive saliva samples can play in the molecular epidemiological analysis of a hepatitis A outbreak. J. Med. Virol. 83:768–775, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Hepatitis A virus (HAV) is the prototype of the genus Hepatovirus and belongs to the Picornaviridae family [Gust et al., 1983; Melnick, 1992]. HAV has a single antigenic serotype [Lemon and Binn, 1983] that determines lifelong immunity as a result of the high conservation of its amino acid sequence and its lack of nucleotide diversity. However, despite this antigenic uniformity, variations can be found by phylogenetic analysis, enabling the classification of human and simian isolates into six genotypes; genotypes I–III are of human origin and IV–VI are of simian origin [Costa-Mattioli et al., 2003; Lu et al., 2004]. Each human genotype (I–III) encompasses two subgenotypes, A and B, and although the genotypes show nucleotide variation greater than 15%, the subgenotypes exhibit 7–7.5% nucleotide variation [Robertson et al., 1992].
Hepatitis A is transmitted by the faecal-oral route, is responsible for the majority of viral hepatitis cases worldwide and is recognized as an important public health disease, especially in intermediate endemic regions [FitzSimons et al., 2010]. Due to public health improvements and better socioeconomic conditions, there has been a decline in HAV endemicity over the past few decades in Brazil [Tanaka, 2000; Villar et al., 2004; Morais et al., 2006; Vitral et al., 2006]. This decline decreases the incidence of natural immunization and creates a potential for massive outbreaks in closed communities such as schools and daycare centers [Villar et al., 2002; de Paula et al., 2002; Morais et al., 2006]. These institutions play an important role in the HAV chain of transmission due to the asymptomatic nature of the infection in children younger than 6 years old, which may lead to silent dissemination of the disease among susceptible adults [Desenclos and MacLafferty, 1993; Venczel et al., 2001; Poovorawan et al., 2005].
Molecular epidemiology has shown that HAV subgenotype IA is the most prevalent globally, while subgenotype IB predominates in the Middle East [FitzSimons et al., 2010]. South American human HAV isolates have been characterized almost exclusively as subgenotype IA with the exception of Brazil, where subgenotype IB has also been reported in hepatitis A outbreaks [Costa-Mattioli et al., 2001; de Paula et al., 2002] and in sporadic cases [de Paula et al., 2004; Fiaccadori et al., 2006; Villar et al., 2006b]. In addition, the co-circulation of both subgenotypes IA and IB in hepatitis A outbreaks has also been reported [Costa-Mattioli et al., 2001; de Paula et al., 2002].
Hepatitis A is diagnosed using serum samples, but many studies have shown the applicability of saliva for diagnosing HAV in sporadic incidents and outbreaks [Stuart et al., 1992; Ochnio et al., 1997; Amado et al., 2006; Oba et al., 2000]. Saliva sample collection is safer and cheaper than blood collection in epidemiologic field studies and can facilitate studies of HAV outbreaks.
To date, two studies have reported the detection of HAV RNA in saliva samples [Mackiewicz et al., 2004; Amado et al., 2008], but to our knowledge the use of saliva samples has not been described in molecular epidemiological studies of HAV outbreaks. Therefore, the feasibility of using saliva specimens for molecular epidemiological studies was evaluated among children infected during a hepatitis A outbreak at a daycare center in Rio de Janeiro, Brazil.
MATERIALS AND METHODS
Epidemiological Research
During October 2006, four cases of acute hepatitis A (three children and one adult) were reported to the Brazilian Reference Centre for Viral Hepatitis (BRCVH), and detailed demographic and risk factor information for hepatitis A infection was obtained. The only common risk factor among these cases was attendance at a daycare center located in Rio de Janeiro City, Brazil. Therefore, an investigation of this possible hepatitis A outbreak was conducted. Hepatitis A cases were defined as a positive serological test for IgM anti-HAV antibodies. Demographic and risk factor information was collected using a questionnaire. Risk factors for HAV infection were defined as having school or household contact with a case, the absence of a sewage system at home and the consumption of non-treated water. The study was performed in compliance with the Ethics Committee of the Oswaldo Cruz Foundation guidelines (CONEP-Fiocruz, number 7071) and in accordance with the ethical standards of the Declaration of Helsinki. Informed patient and/or parental consent was obtained for all subjects included in this study.
Sample Collection and Processing
Matched blood and saliva samples were obtained from 124 individuals. Blood was collected by venipuncture into sterile tubes, and the serum was separated by centrifugation and stored at −20°C. Saliva samples were collected using OraSure collection devices (Epitope Incorporated, Beaverton, OR) according to the manufacturer's instructions and separated by centrifugation at 2,500 × g for 15 min at room temperature. All saliva and serum specimens were processed on the day of collection and stored at −20°C until testing.
Water samples were also collected to investigate the role of water sources in disseminating HAV during the outbreak. Ten different sources of water, including three samples from water reservoirs, four samples of tap water, and three samples from drinking fountains were collected at the daycare center. After collection, the tap water was dechlorinated with sodium thiosulphate at a final concentration of 50 mg/L. A total of 500 ml of each sample was processed as described previously [Villar et al., 2006b] to concentrate the virus and obtain a suspension with a final volume of 800 µl.
Anti-HAV Detection in Serum and Saliva Samples
IgM and total anti-HAV enzyme immunoassays (EIA) were performed on serum samples (n = 124) using a commercial kit (Biokit, Barcelona, Spain) according to the manufacturer's instructions. For the saliva samples (n = 124), undiluted samples (100 and 15 µl for IgM and total anti-HAV, respectively), were tested using a modified EIA (Biokit), and the sensitivity of modified total and anti-HAV IgM EIAs were 98% and 96%, respectively [Amado et al., 2008].
Detection and Quantitation of HAV in Serum, Saliva, and Water Samples
Viral RNA was extracted from 140 µl each of serum, saliva, and water samples using the QIAmp viral RNA kit (Qiagen, Courtaboeuf, France) according to the manufacturer's instructions and was eluted with the a Qiamp spin column using 60 L of RNAse/DNAse-free water; the eluted RNA was stored at −70°C until use.
A 247-bp fragment encompassing the VP1-2A junction of the HAV genome was amplified by nested reverse-transcription polymerase chain reaction (RT-PCR) using primers and conditions described previously [de Paula et al., 2003b]. RT-PCR was performed with 10 µl extracted RNA using Superscript II H− reverse transcriptase (Invitrogen, San Diego, CA) and random hexamer primers according to the manufacturer's instructions. For the saliva samples, the cDNA and Taq polymerase volumes were increased twofold to increase the sensitivity of the assay [Amado et al., 2008].
HAV RNA was quantified from 5 µl of cDNA using the TaqMan® real-time PCR assay (Applied Biosystems, Foster City, CA). A recombinant clone of the conserved 5′ non-coding region (NCR) of HAV (strain HAF-203) [Gaspar et al., 1993] was used to establish the standard curve, and specific primers for the 5′ NCR of HAV and a single-labeled 5′ FAM probe were used as described previously [de Paula et al., 2007]. The detection limits of the assays on serum, saliva, and water samples were 14, 140, and 60 copies/ml, respectively [Villar et al., 2006a; de Paula et al., 2007; Amado et al., 2008]. Particular care was taken to prevent the cross-contamination of samples during molecular assays. The tests were conducted in separate rooms, and three negative samples were included for every PCR run to control for contamination of the first and second rounds of PCR.
Genetic Analysis of HAV
HAV RNA-positive samples were genotyped by direct sequencing of the purified second PCR products using internal primers and the ABI PRISM BigDye Terminator Cycle Sequencing Kit (Applied BioSystems) in a Gene Amp PCR System 9700 sequencing apparatus (Applied Biosystems, Foster City, CA) [Otto et al., 2008]. A 168-bp sequence of VP1/2A was used to construct a neighbor-joining tree based on Kimura-2 parameter distances in the program MEGA [Kumar et al., 2001]. The HAV genotype was determined by including reference sequences from Genbank, including the genotype IA strain HAS-15, the IB strains HM-175 (Australia), and HAF-203 (Brazil), the IIA strain SLF88 (Sierra Leone), the IIB strain CF-53 (France), and the IIIA strain GA76. Thirteen HAV strains isolated from different Brazilian states and South American counties available from GenBank were also included in this analysis. The GenBank accession numbers for the VP1/2A nucleotide sequences reported here are HM357765–HM357788.
Mixed HAV infection in patients with two subgenotypes was investigated by cloning HAV PCR products (247 bp) into the pCR4.1-TOPO vector (TOPO TA cloning kit, Invitrogen) according to the manufacturer's instructions. After the growth of transformed Escherichia coli, recombinant plasmids were purified by using the QIAprep Spin Miniprep Kit (Qiagen, Hilden, Germany), and both strands of the insert DNA were sequenced using forward and reverse M13 primers (TOPO TA cloning kit, Invitrogen) [de Paula et al., 2003a].
Data Analysis
Epidemiological patient data were included in a logistic regression analysis to identify independent factors associated with the detection of anti-HAV IgM in serum and saliva samples after adjustment for confounding variables. To assess continuous variables, Student's t-test or the Mann–Whitney U-test were used as appropriate; a two-tailed P < 0.05 was considered to be statistically significant. Analyses were conducted using the Statistical Package for the Social Sciences (SPSS for windows, release 8.0; SPSS Inc., Chicago, IL).
RESULTS
Epidemiology
Sixty-five children aged 1–4 years (3.1 ± 0.7 years), 21 staff members (teachers and cooks) aged 22–57 years (37.1 ± 10.7 years), and 38 household contacts aged 1–11 years (5.7 ± 2.7 years) were enrolled in this study.
The majority of the studied population (61%) presented a low socioeconomic status, and although most subjects reported having access to sewage disposal (96%), 46% of them reported living near open sewage, and among them 11% had direct contact with this sewage water; 27% reported that they consumed untreated water. At least 31% reported previous contact with hepatitis A-infected persons. However, the epidemiological data showed that none of these risk factors were related to hepatitis A infection (P > 0.05).
Hepatitis A infection was confirmed in 52 (42%) individuals that tested positive for anti-HAV IgM in serum samples and in 51 (41%) subjects that were positive for the antibodies in saliva samples. Among these individuals, 64% were asymptomatic. The frequency of acute infection was significantly higher in children up to 3 years old than in household contacts (8/52) or staff members (1/52) (χ2 = 52.2; P = 0.002). Total anti-HAV was detected in 77% (96/124) of the saliva samples and in 78% (97/124) of the serum samples. Forty-seven percent (46/97) of the patients with positive serum samples had been infected in the past, and 21% (26/124) of all the test subjects remained susceptible to hepatitis A infection (Table I).
| Characteristic | Daycare children (n = 65) | Staff members (n = 21) | Household contacts (n = 38) | Total (n = 124) |
|---|---|---|---|---|
| Gender | ||||
| Male | 31 (48%) | 2 (9.5%) | 21 (55%) | 54 (44%) |
| Female | 34 (52%) | 19 (90.5%) | 17 (45%) | 70 (56%) |
| Age distribution | ||||
| 0–5 | 65 (100%) | 0 | 16 (42%) | 81 (65%) |
| 6–10 | 0 | 0 | 21 (53%) | 21 (17%) |
| 11–15 | 0 | 0 | 1 (3%) | 1 (0.8%) |
| 16–20 | 0 | 0 | 0 | 0 |
| 21–30 | 0 | 7 (33%) | 0 | 7 (6%) |
| 31–40 | 0 | 6 (29%) | 0 | 6 (5%) |
| >40 | 0 | 9 (43%) | 0 | 9 (7%) |
| Hepatitis A statusa | ||||
| Acute | 43 (66%) | 1 (5%) | 8 (21%) | 52 (42%) |
| Susceptible | 11 (17%) | 1 (5%) | 14 (37%) | 26 (21%) |
| Immune | 22 (34%) | 19 (90%) | 16 (42%) | 57 (46%) |
| HAV-RNA positiveb | 20 (31%) | 0 | 5 (13%) | 25 (21%) |
| Risk factors | ||||
| Close contact with HAV cases | 16 (25%) | 17 (81%) | 6 (16%) | 39 (31%) |
| Sewage disposal | 62 (95%) | 21 (100%) | 36 (95%) | 119 (96%) |
| Contact with sewage water | 6 (9%) | 0 (0%) | 8 (21%) | 14 (11%) |
| Consumption of non-treated water | 22 (34%) | 3 (14%) | 9 (24%) | 34 (27%) |
- a Hepatitis A status: Acute, IgM and total anti-HAV positive; susceptible, IgM and total anti-HAV negative; Immune, total anti-HAV positive.
- b HAV-RNA positive by nested-PCR in serum samples.
Detection of HAV-RNA in Serum and Saliva Samples
HAV RNA levels were analyzed in serum and saliva samples from acute hepatitis A patients (anti-HAV IgM-positive; n = 52) and from susceptible patients (IgM and total anti-HAV-negative; n = 26). HAV RNA was detected by nested PCR in 42% (22/52) of the serum samples and 51% (26/51) of the saliva samples from acute hepatitis A cases. Among IgM and total anti-HAV-negative patients, HAV RNA was detected in three sera and in three saliva samples. Overall, HAV was detected by nested PCR in 37.7% (29/77) of the saliva samples and 32% (25/78) of the serum samples. The rate of HAV RNA detection was higher in children (n = 20) when compared to household contacts (n = 5) and staff members (none). HAV was detected successfully by real-time PCR in 48.7% (38/78) of the serum and 58.9% (46/78) of the saliva samples. Among them, 3 serum and 15 saliva samples were from IgM/total anti-HAV-negative subjects. The hepatitis A viral loads in saliva samples ranged from 1.0 × 102 to 1.4 × 104 copies/ml (mean of 1.7 ± 3.24 × 103 copies/ml), which was similar to the serum viral loads that ranged from 1.2 × 102 to 3.2 × 104 copies/ml (mean of 2.8 ± 6.46 × 103 copies/ml). Of the water samples collected, HAV-RNA was detected in one sample (1/10) from a drinking fountain used exclusively by children of the daycare center.
Genetic Analysis of HAV Isolates
Sequence information was obtained from HAV RNA isolates of 23 serum and 26 saliva samples (three saliva samples were untypeable by sequencing, probably because of the low viral load in these samples). All isolates belonged to genotype I. Of these, 16 isolates from sera were assigned to genotype IA and 7 isolates to genotype IB. Among the sequences isolated from saliva, 9 were classified as genotype IA and 17 were classified as genotype IB. As seen in Figure 1, the majority of the strains of this outbreak that shared the same subgenotype were clustered together. After a pairwise comparison, the HAV isolates of genotype IA showed slightly higher identity (93.6–100%; mean: 96.8%) than the isolates of genotype IB (90.5–100%; mean: 95.4%). The genotype of the virus in the salivary specimens was the same as that found in the matched serum specimens of six patients. The remaining six patients had differing subgenotypes in their serum and saliva specimens. This unusual pattern of a mixed HAV population in these patients was investigated further by molecular cloning followed by nucleotide sequencing.
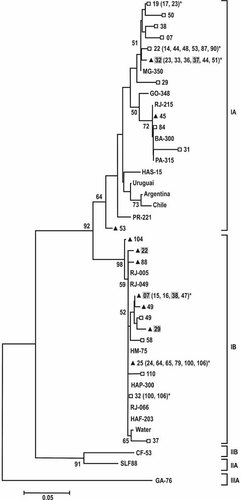
Phylogenetic tree of 68 HAV isolates, constructed with the neighbor-joining method, and based on 168 nucleotides (positions 3024 ± 3191). Roman numerals designate the respective genotype groupings, whereas (A) and (B) designate subgenotypes. Bootstrap percentages were calculated from 2,000 replicates and the numbers higher than 50 are shown. The horizontal bar at the top represents an evolutionary distance of 0.05. The following symbols indicate the type of clinical specimen of the HAV isolate in this work: serum (□), saliva (▴), and the identical sequences with 100% of homology (*). Gray square indicates saliva samples of the patients mixed infected with subgenotypes IA and IB. References sequences from GenBank included subgenotypes IA: HAS-15 (X15464), IB: HM-175 (M14707, Australia), and HAF-203 (AF26896, Brazil), IIA: (L07729, Sierra Leone), IIB: CF-53 (L07693, France), and IIIA: GA76 (L07668). The sequences from Brazilian states: RJ (Rio de Janeiro) (AY322851) (AY322850) (AF410381) (AF410387) (AF538727), MG-350 (Minas Gerais) (AY323002), GO-348 (Goiás) (AY322994), PA-315 (Pará) (AY322923), BA-300 (Bahia) (AY322898), PR-221 (Paraná) (AY323046) and from Uruguay (AJ306387), Chile (AJ306383), and Argentine (AJ306374) has been reported by De Paula et al. [2004]. *For clarification, the number of sequences shown in the clusters was reduced.
The HAV sequence isolated from the drinking fountain clustered into subgenotype IB and showed 97.5–99.3% identity with the saliva sequences of subgenotype IB that were isolated from the children of the daycare center.
Phylogenetic Analysis of the HAV Isolates
To gain insight into the degree of genetic heterogeneity of this outbreak, the HAV sequences isolated from this study were compared with 10 isolates from other regions of Brazil and three other South American countries whose sequences had been determined previously [de Paula et al., 2004]. Within subgenotype IA, most of the sequences from this work were 96.3–100% homologous with previous isolates from sporadic cases in other Brazilian states (MG, GO, BA, and PA). Among subgenotype IB, the isolates from this study were 98.1–100% homologous with sequences isolated previously in outbreaks in Rio de Janeiro and clustered on the same branch of the phylogenetic tree. When compared to the HAV isolates from Uruguay, Argentina, and Chile, a sub-cluster different from that of the genotype IA isolates of this study appeared (Fig. 1).
Mixed Infection With HAV Subgenotypes IA and IB
For the samples in which mixed HAV infections were initially detected, nested PCR, and nucleotide sequencing were repeated to test for contamination and to confirm the results; however, the presence of both subgenotypes was still observed in these matched specimens. To investigate the incidence of these mixed infections with different subgenotypes detected in serum and saliva, molecular cloning of these PCR products from four patients (07, 22, 29, and 37) was undertaken, followed by multiple sequencing of the different clones. Clones from two patients (32 and 38) were untypeable by sequencing, and a total of 32 clones were selected at random for further characterization by nucleotide sequencing. Table III shows that all the clones derived from saliva samples (n = 14) belong to subgenotype IB and display close identity to one another at the nucleotide level. However, all the clones derived from their corresponding matched sera (n = 19) belong to subgenotype IA and display heterogeneity.
| Subject | Serum | Saliva | ||
|---|---|---|---|---|
| Ampliconsa genotype | Clones homology (n) | Ampliconsa genotype | Clones homology (n) | |
| 07 | IA | 97.5–100% (5) | IB | 100% (3) |
| 22 | IA | 99.4–100% (8) | IB | 100% (2) |
| 29 | IA | 90.5–95.2% (3) | IB | 96.5–99.1% (5) |
| 37 | IA | 92.3–95.2% (3) | IB | 100% (4) |
- a The genotyping was based on PCR amplicons of 168 bp of the VPI/2a region of the HAV genome.
DISCUSSION
Epidemiological investigations of hepatitis A outbreaks have traditionally focused on demographic and risk factor information, and the molecular characterization of HAV from serum samples has only been included more recently. This report describes a molecular epidemiological study of a hepatitis A outbreak that identifies and characterizes the HAV genome from saliva specimens. Child care centers have been described as an important source of HAV transmission [Venczel et al., 2001; Morais et al., 2006], as the spread of HAV depends primarily on poor hygienic conditions (e.g., close contact between toddlers who are still in diapers and young children). According to previous studies, a high frequency of children under 5 years of age in a center is a significant risk factor (P < 0.05) for the occurrence of a hepatitis A outbreak [Smith et al., 1997; Robertson et al., 2000; Morais et al., 2006]. In this study, direct contact with previous hepatitis A cases was not found to be a significant risk factor for HAV, most likely due to the subclinical nature of the disease among children, as 64% of the cases were asymptomatic. As in other studies, children with asymptomatic infections were an important source of hepatitis A infection for household contacts with no recognized risk factors during the daycare center outbreak [Staes et al., 2000; Nainan et al., 2005].
The association between water consumption and hepatitis A dissemination was investigated, and HAV was detected in a water sample from a drinking fountain. However, it was not detected in samples from water reservoirs that supply this drinking fountain. A matched comparison revealed that HAV isolated from the drinking water shared a close identity (97.5–99.3%) with HAV isolated from the saliva of children linked to the outbreak. These findings suggest that the drinking fountain could have been contaminated by the child's hands or HAV-containing saliva, although we are unable to prove this hypothesis. This epidemiological analysis indicates that the drinking fountain might be a vehicle of HAV transmission (potential reservoir), which would exclude water contamination as a source of HAV infection. The epidemiological investigation was not able to reveal a statistically significant risk factor or common sources of HAV, most likely due to the subclinical nature of the disease during the outbreak and the homogeneity of the demographic data of the studied population.
In this study, subgenotype IA was the most prevalent HAV subgenotype detected, which is in accordance with previous studies conducted on the American continent [Robertson et al., 1992; Costa-Mattioli et al., 2001; Diaz et al., 2001; Mbayed et al., 2002]. The predominance of HAV subgenotype IA circulating in South America has suggested an endemic circulation of this subgenotype in these countries [Costa-Mattioli et al., 2001]. In agreement with previous studies conducted in the State of Rio de Janeiro [de Paula et al., 2002; Devalle et al., 2003; de Paula et al., 2003a], this study identified the co-circulation of subgenotypes IA and IB in the daycare center outbreak. Many isolates from this study were closely related genetically (or even identical; 96.3–100% identity) to isolates originating in previous studies in Brazil (Fig. 1). These findings suggest an endemic pattern of Brazilian HAV strains circulating during outbreaks.
Comparison of the HAV genotypes of matched serum and saliva specimens revealed six patients whose viral genotype in their salivary specimen was the same as that in their serum specimen, and the remaining six patients had differing subgenotypes in their serum and saliva specimens. These patients may have had a mixed infection, but because different subgenotypes predominantly infect two different body fluids, it is possible that when sequencing was performed, only the predominant subgenotype prevailed. Alternatively, an indeterminate sequence could emerge as the mixed sequences from the different viruses appear together, which might explain the high number of isolates that could not be genotyped [Roy et al., 1998].
Two conditions seem to be necessary for this phenomenon; the co-circulation of different subgenotypes in the outbreak, and the contact of a susceptible subject with at least two sources of infection in a short period of time. Although mixed HAV infections are uncommon, one was also reported by de Paula et al. [2003b] in a patient exposed to a hepatitis A outbreak at a childcare center, indicating that multiple HAV exposures appear to be inevitable within this patient group during daycare center outbreaks.
The molecular cloning conducted to investigate the mixed infections showed that the distribution of genotypes in saliva was different from that in serum, because all the clones from saliva were classified as IB whereas all those in serum were IA. Coexisting virus subpopulations at different sites have been documented extensively for HIV type I [Shapshak et al., 1999; Papathanasopoulos et al., 2003] and HCV [Laskus et al., 2000; Chen et al., 2003], suggesting that this strategy may allow the virus to adapt rapidly to changes in its environment [Cristina and Costa-Mattioli, 2007]. Sánchez et al. [2003] reported that the presence of different HAV strains at the involved sites might be related to the generation of a mutant spectrum in the course of each HAV replication, probably due to different selective pressures.
However, this unexpected discrepancy between saliva and serum provided some interesting data that challenge the current understanding of the source of salivary HAV RNA. This study showed that HAV was more often present in the saliva of acutely infected patients (37.7%) than in the serum (29%). Therefore, in addition to the transudation of HAV-containing fluid from the general circulation into the saliva through gengivo-crevicular fluid, there may be other sources of HAV in saliva, including active viral replication at the site of salivary secretion in some individuals, as has been suggested for hepatitis C virus infections [Takamatsu et al., 1992; Roy et al., 1998; Arrieta et al., 2001]. HAV negative-strand RNA (intermediate replicative) could be detected in the salivary glands of non-human primates experimentally infected with HAV, demonstrating active HAV replication at this site [Amado et al., 2010]. Therefore, active HAV replication in the salivary glands would perhaps explain the discrepant subgenotypes between matched serum and saliva specimens. However, negative-strand RNA was not investigated in saliva samples in this study, because this marker is only present inside the cell where replication is occurring and therefore would not be detected in oral fluid samples. Another explanation for these mixed infections could be a recent exposure to the HAV subgenotype IB from the drinking fountain, possibly a few hours prior to sample collection, which then contaminated the saliva samples. However, all such children were anti-HAV IgM-positive on the collection day, indicating that they were infected a few weeks before the collection. In addition, subjects infected with the subgenotype IA in saliva and IB in matched serum samples were also identified, indicating that the virus detected in saliva samples was not from the drinking fountain source. Further studies are needed to determine whether HAV RNA-positive saliva can actually transmit infection.
In conclusion, saliva was shown to be useful for molecular epidemiological purposes during an outbreak and is the more appropriate specimen for HAV detection during outbreaks. The occurrence of co-infections with different subgenotypes was confirmed in this HAV outbreak; however, further studies are necessary to investigate the biological and clinical meaning of the uneven distribution of HAV subgenotypes observed in the matched serum and saliva specimens from patients with mixed infections.
Acknowledgements
We thank Dr. Flor Pujol for revising the manuscript; Marcia Paschoal do Espírito-Santo for the molecular analysis; Liliane Morais, Debora RL. dos Santos, Elisangela Ferreira, and Ilton Santana for technical assistance; as well as the daycare center staff for allowing us to investigate this hepatitis A outbreak. We also thank Dr. Adilson José de Almeida for his help in conducting statistical analyses and the genomic platform—PDTIS/FIOCRUZ (RPT01A) to carry out the genomic sequencing.




