Mother-to-infant transmission of hepatitis B virus: A Chinese experience†
Shao ZJ and Zhang L contributed equally to the work.
Abstract
Over 90% of infants infected with hepatitis B virus (HBV) caused by mother-to-infant transmission will evolve to carrier status, and this cannot be prevented until widespread administration of the HB vaccine and hepatitis B immune globulin (HBIG) is implemented. This prospective study of 214 infants born to HBsAg-positive mothers was carried out to determine if either perinatal or intrauterine HBV transmission could be effectively prevented with HBIG and the HB vaccine. Peripheral blood was collected from mothers and from newborns before they received HBIG and the HB vaccine, as well as at 0, 1, 7, 24, and 36 months after birth. Infants born with an ratio of signal to noise(S/N) value of >5 for HBsAg (ABBOTT Diagnostic Kit) were defined as mother-to-infant transmission cases, those with an S/N between 5 and 50 were classified as perinatal transmission cases, and those with an S/N >50 were considered intrauterine transmission cases. Mother-to-infant transmission occurred in approximately 4.7% (10/214) of the infants; the perinatal transmission and intrauterine transmission rates were 3.7% (8/214) and 0.9% (2/214), respectively. The risk of mother-to-infant transmission increased along with maternal HBeAg or HBVDNA levels. After 36 months of follow-up, all perinatal cases became HBsAg-negative, whereas all intrauterine transmission cases evolved into carrier status. These results indicate that infants infected via intrauterine transmission cannot be effectively protected by HBIG and HB vaccine. J. Med. Virol. 83:791–795, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Hepatitis B virus (HBV) infections are a global health problem. One-third of the world population has serological evidence for current or past HBV infection, and 15–25% of the 350 million people with chronic HBV face a risk of early death from HBV-related liver diseases, such as end-stage liver cirrhosis or hepatocellular carcinoma [Chang, 2007; Wilt et al., 2008]. Mother-to-infant HBV transmission contributes significantly to the persistence of the high number of HBV carriers because the risk for newborns to develop a chronic infection is greater than 90%, whereas no more than 5% of the adult population develops chronic HBV infection [Burk et al., 1994; Zhu et al., 1997]. Thus, infected newborns often have poor prognoses. Carrier status during childhood facilitates intrafamilial transmission, particularly to siblings, as well as transmission in preschools and daycare centers.
Since 2002, HBIG and HB vaccine have been used to prevent perinatal HBV transmission in China. The aim of this prospective study was to determine whether mother-to-infant transmission of HBV in China could be effectively prevented with HB vaccine and HBIG.
METHODS
Study Population
HBsAg-positive mothers and their infants were recruited for the study; pregnant women aged 20–40 years who underwent regular checkups for serum HBsAg at the Maternal and Neonatal Hospital of Shaanxi Province, Xi'an, China from September 2002 to October 2005 were screened, and their infants were followed until the end of 2008. Blood specimens were taken from mothers and their newborns at delivery. To prepare serum, blood samples were collected from the newborns via the femoral artery and stored at −20°C until use. The parents or legal guardians of the infants were contacted by the Maternal and Neonatal Hospital via telephone, email, or letters and were interviewed by an experienced investigator using a structured questionnaire to collect demographic data at each follow-up visit. Infants were seen at 1, 7, 24, and 36 months, at which time blood samples were collected by venipuncture.
Passive and active immunoprophylaxis was given to infants according to the Chinese HBV vaccination schedule. Within 12 hr of delivery, a 200-IU-dose of hepatitis B immune globulin (HBIG, Shanghai Institute of Biological Products, Shanghai, China) and a 10-µg-dose of recombinant yeast-derived hepatitis B vaccine (manufactured by Kangtai Biological Products Co. Ltd, Shenzhen, China) were given intramuscularly after blood collection. Subsequently, 10-µg doses of hepatitis B vaccine were given to the infants at 1 and 6 months after birth according to the manufacturer's protocol.
The study protocol was approved by the Ethics Committee for Medical Research of the Fourth Military Medical University, and written informed consent was obtained prior to the study.
Determination of HBV Biomarkers
All maternal serum specimens were tested by ELISA for HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc (Kehua Co., Shanghai, China). Maternal HBV DNA analysis was performed using the COBAS AMPLICOR HBV monitor test (Roche Molecular Systems). Viral loads above the linear range were determined by dilution as recommended by the manufacturer. HBV DNA was extracted from the serum of HBsAg-positive infants and their mothers and amplified by polymerase chain reaction (PCR) as described in our previous report[Shao et al., 2007]. Serum specimens were tested by ELISA for HBsAg (Abbott Laboratories, Abbott Park, IL, USA), anti-HBs, HBeAg, and anti-HBe plus anti-HBc [total anti-HBc; if positive, also for anti-HBc IgM (Kehua Co., Shanghai, China)] if enough serum was available.
For HBsAg ELISA (Abbott Laboratories, Abbott Park, IL, USA), the signal to noise ratio (S/N) is used to reflect infant HBsAg titers. An S/N of 5 was used as the cut-off value for HBV diagnosis in infants. An S/N between 5 and 50 was considered HBV perinatal transmission, and cases with an S/N>50 were designated intrauterine transmission.
Statistical Analysis
Fisher's exact test was used to detect associations between grouped maternal viral load, maternal HBeAg positivity, and transmission rates. Statistical analysis was performed using the Statistical Package for the Social Sciences (version 10.0, SPSS, Chicago, IL, USA). Statistical significance was defined as P < 0.05 (two-tailed).
RESULTS
Characteristics of the Included Infants and their Mothers
A total of 212 HBsAg-positive pregnant women and their 214 infants (including two sets of twins) were recruited between September 2002 and October 2005. All enrolled mothers were Xi'an citizens belonging to the Han ethnic group. Of the 214 infants, 140 were delivered by caesarean section, and 110 were male. Five infants had an APGAR score of less than 5 within 5 min after birth, and five infants were born weighing under 2500 g. Of the 212 mothers, 47 (22.1%) were HBeAg-positive, none were anti-HB-positive, all were anti-HBc-positive, and 5 were IgM anti-HBc-positive. Of the 212 HBsAg-positive pregnant women, 64 (30.2%) had detectable HBV DNA. The viremic mothers were arbitrarily stratified into three groups based on their HBV DNA levels: 21 had levels between 103 and 105 copies/mL (low viral load), 32 had levels between 105 and 108 copies/mL (middle viral load), and 11 had levels >108 copies/mL (high viral load). Of mothers with detectable HBV DNA, 43(20.1%) were HBeAg-positive. HBeAg positivity was strongly correlated with high viral load (P < 0.001), and all patients with HBV DNA levels >108 copies/mL were HBeAg-positive (Table I).
| HBV DNA level | HBeAg-negative mothers (n = 165) | HBeAg-positive mothers (n = 47) |
|---|---|---|
| <103copies/mL | 144(87.3%) | 4(8.5%) |
| 103–105copies/mL | 11(6.6%) | 10(21.3%) |
| 105–108copies/mL | 10(6.1) | 22(46.8%) |
| >108copies/mL | 0(0%) | 11(23.4%) |
| Totals | 165 | 47 |
S/N values ranged from 0.98 to 109 among the 214 infants. Seventy-one infants were born with an S/N >5 for HBsAg by ABBOTT diagnostic reagent, and ten were diagnosed as mother-to-infant transmission cases. There were no differences in maternal education levels (10.40 ± 3.59 vs. 10.83 ± 3.23 years), maternal age at interview (27.37 ± 3.73 vs. 28.08 ± 3.48 years) or neonatal weight (3250.00 ± 336.45 vs. 3035.64 ± 860.37 g) between newborns with mother-to-infant HBV transmission and those without. Two infants with HBsAg S/Ns of 50.93 and 109.71 were identified as intrauterine HBV transmission cases. Perinatal transmission (HBsAg S/N under 50) was identified in 8 of the 214 infants (3.7%).
Compliance of Mothers and their Infants During Follow-Up
Of the 214 infants, 57 (26.6%), 158 (73.8%), 144 (67.3%), and 38 (17.8%) attended follow-up visits at 1, 7, 24, and 36 months of age, respectively. Thirty-one infants did not attend any of the scheduled follow-up visits. The main reasons for failure to follow-up were fear of venipuncture, concern about cross-infection, living too far from the hospital, a change in telephone number, and wrong address/relocation. A total of 122 infants entered the cohort from month 7 to month 24 but did not attend the month 36 visit. Among the remaining 48 infants, attendance varied at months 1, 7, 24, or 36. There were no differences in maternal age, maternal educational level, or neonatal weight at birth between infants who attended follow-up visits and those who failed to follow-up at various time points (Table II).
| Characteristics | Month 1 | Month 7 | Month 24 | Month 36 | ||||
|---|---|---|---|---|---|---|---|---|
| Follow-up | Failed to follow up | Follow-up | Failed to follow up | Follow-up | Failed to follow up | Follow-up | Failed to follow up | |
| Number | 57 | 157 | 158 | 56 | 144 | 70 | 38 | 176 |
| Birth weight (Kg) | 3124.2 ± 650.8 | 3132 ± 550.2 | 3244.6 ± 441.8 | 3067 ± 550.3 | 3274.4 ± 450.8 | 3357.7 ± 450.1 | 3489 ± 450.2 | 3143 ± 550.1 |
| Number of male infants | 32(56.1%) | 77(49.0%) | 64(40.5%) | 45(80.3%) | 78(54.2%) | 32(45.7%) | 23(60.5%) | 85(48.3%) |
| Maternal age at delivery | 27.5 ± 4.5 | 27.6 ± 6.9 | 28.7 ± 5.6 | 27.9 ± 7.5 | 27.1 ± 4.1 | 28.2 ± 5.2 | 28.6 ± 5.5 | 26.2 ± 5.6 |
| No. | Maternal statusa | Maternal HBV DNA copies | Neonatal HBsAg titierb | Neonatal status at birth | Month 1 | Month 7 | Month 24 | Month 36 |
|---|---|---|---|---|---|---|---|---|
| 9 | 1.4.5 | <103/mL | 5.35 | 1.4.5 | 2.4.5 | 2.4.5 | 2.5 | 2 |
| 17 | 1.3.5.6.7 | 5 × 106/mL | 9.42 | 1.3.5.6 | 2.5 | 2.5 | 2.5 | 2.5 |
| 39 | 1.3.5.7 | 2 × 107/mL | 7.06 | 1. 5.6 | 5 | 2.5 | 2.5 | 2 |
| 54 | 1.3.5.7 | 4 × 108/mL | 103.1 | 1.3.5.7 | 1.3.5.7 | 1.3.5 | 1.3.5 | F |
| 97 | 1.4.5 | 3 × 104/mL | 5.76 | 1.4.5 | 2.4.5 | F | F | 2.5 |
| 154 | 1.3.5.7 | 5 × 105/mL | 50.93 | 1.3.5.6.7 | 1.3.5.7 | 1.3.5.7 | 1.3.5 | 1.3.5 |
| 178 | 1.3.5.7 | 3 × 106/mL | 7.54 | 1.3.5 | 2.5 | F | F | 2.5 |
| 197 | 1.4.5 | <103/mL | 5.87 | 1.4.5 | 4.5 | 2.4.5 | 2.4.5 | 2 |
| 200 | 1.3.5.7 | 7 × 107/mL | 8.83 | 1.5 | F | 2.5 | 2 | 2 |
| 210 | 1.4.5 | <103/mL | 7.34 | 1.4.5 | 4.5 | 2.5 | 2.5 | 2 |
- 1 = HBsAg 2 = anti-HBs, 3 = HBeAg, 4 = anti-HBe, 5 = anti-HBc, 6 = IgM anti-HBc, 7 = HBVDNA, F = fail to follow up
- a Maternal bloods were collected during labor before delivery.
- b S/N value.
Mother-to-Infant Transmission Cases
Mother-to-infant transmission was detected in 10 infants. In two of these cases, high HBsAg titers were detected in their peripheral serum at birth (50.93 in infant No. 54, 109.71 in infant No. 154), and they developed HBV carrier status during the follow-up period. Moreover, they were continuously positive for HBV DNA, HBsAg, and HBeAg during the follow-up period. The other eight identified perinatal transmission cases became negative for HBV DNA, HBsAg, and HBeAg during the follow-up period: six of these newborns were born to HBeAg-positive women, and four of the six were born to HBeAg-positive mothers. The risk of transmission was significantly higher from HBeAg-positive mothers than HBeAg-negative mothers (6/41 vs. 4/163, odds ratio = 5.96 (1.60–22.12), P < 0.001) as well as from mothers with detectable HBV DNA levels compared with mothers with non-detectable HBV DNA levels (7/57 vs. 3/145, odds ratio = 5.94 (1.48–22.75), P < 0.001). The risk of HBV mother-to-infant transmission increased with maternal HBV DNA levels (3/145 low viral load group vs.1/20 middle viral load group vs.5/27 high viral load group vs. 1/10 very high viral load group, P < 0.001).
Dynamic Changes in Other HBV Biomarkers in Infants
Anti-HBs were not detected in any infants at birth. The rate of anti-HB positivity was 82.5% (47/57), 91.7% (145/158), 70.8% (102/144), and 73.7% (28/38) at 1, 7, 24, and 36 months of age, respectively. The trend of anti-HB over time was characterized by a steep rise in the first month followed by a gradual decrease, eventually reaching a plateau at 24 months of age (Fig. 1).
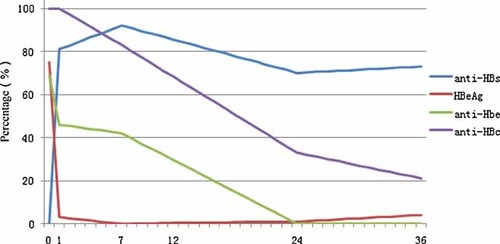
Dynamic changes in serum anti-HBs, HBeAg, anti-HBe, and anti-HBc after delivery and during the 36 months of follow up.
Forty-seven of the 212 mothers were HBeAg-positive, and 35 of their infants (74.5%) were HBeAg-positive at birth. Of the HBeAg-positive infants, all became HBeAg-negative at the first month, except for two infected via intrauterine transmission, and remained so thereafter. No HBeAg was detected in the 167 infants born to mothers who were HBeAg-negative at birth.
Anti-HBe was detected in 152 mothers (152/214, 71.0%), and all infants born to anti-HBe-positive mothers were anti-HBe-positive at birth. However, anti-HBe was not detected in the 62 infants born to the 60 anti-HBe-negative mothers. Anti-HBe was detected in 27/52 (47.4%) infants at month 1 and 52/116 (44.8%) at month 7, but not at month 24 (0/144) or month 36 (0/38). No infants became anti-HBe-positive during the follow-up period.
Anti-HBc was detected in all infants born to anti-HBc positive mothers both at birth (214/214) and at month 1 (57/57). The anti-HBc positivity rate was 84.8% (134/158) at month 7, 34.0% (49/144) at month 24, and 21.1% (8/38) at month 36. However, two of the infants identified as HBV carriers at birth were positive for IgM anti-HBc, but this disappeared within 7 months after delivery.
DISCUSSION
HBV infection is a leading cause of acute and chronic liver disease in children and adolescents worldwide [Chang, 2007; McMahon, 2009]. In areas of high endemicity in China, especially in northwestern China, the carrier rate for hepatitis B surface antigen (HBsAg) is as high as three times the national average of China (http://www.chinacdc.cn/n272442/n272530/n294176/n339985/index.html). Treatment strategies for children with chronic hepatitis B have focused on inducing HBeAg seroconversion and inhibiting viral replication to prevent active liver damage. However, the initiation of antiviral therapy in children is challenging and often controversial [Wilt et al., 2008; Beasley, 2009; McMahon, 2009]. Therefore, prevention is still important for controlling mother-to-infant transmission. HBIG is a plasma product that can prevent hepatitis B if given to an infected individual within 14 days of exposure [Buchanan and Tran, 2010; Gane, 2010]. HBIG is given in conjunction with a three-dose hepatitis B vaccine series after exposure to the blood or sexual body fluids of an HBV-infected person. Beginning in 2002, similar measures were implemented in China; infants born to HBsAg-positive mothers now receive HBIG and hepatitis B vaccination within 24 hr of birth. In this study, all enrolled infants received prophylaxis within 24 hr of birth according to the hospital records. Eight infants with verified perinatal HBV transmission became negative during the follow-up period. In contrast, both infants diagnosed with intrauterine transmission cases evolved to HBV carrier status. This finding is consistent with previous reports that suggest preventive measures play a limited role in eliminating HBV in infants infected via intrauterine transmission [Zhu et al., 2003; Ni, 2010]. In the present study, the rate of mother-to-infant transmission was 4.7% (10/214), and the 0.9% of infants that remained positive for HBsAg were verified to be intrauterine transmission cases. These rates are lower than the 5–17% reported by some Chinese studies with both larger and smaller sample sizes [Xu et al., 1998; Yan et al., 1998; Dz and Yp, 1999].
HBeAg and HBV DNA in maternal serum are considered reliable markers of potential infectivity and may be associated with a high risk of perinatal HBV transmission [; Xu et al., 2002; Shao et al., 2007]. In the present study, the risk of transmission was significantly higher from HBeAg-positive mothers than HBeAg-negative mothers [6/41 vs. 4/163, odds ratio = 5.96 (1.60–22.12)], and the same was true for maternal HBV DNA load [7/57 vs. 3/145, odds ratio = 5.94 (1.48–22.75)], which confirms previous observations[Burk et al., 1994; Zhu et al., 2003; Wang et al., 2005; Shao et al., 2007]. HBV DNA positivity is more accurate than HBeAg to predict infectivity. In the present study, maternal HBV DNA level was closely and positively associated with the incidence of HBV perinatal transmission, which is consistent with previous reports[Burk et al., 1994; Xu et al., 2002; Shao et al., 2007]. The risk of transmission was significantly higher from HBeAg-positive mothers than HBeAg-negative mothers [6/41 vs. 4/163, odds ratio = 5.96 (1.60–22.12), P < 0.001], and significantly higher in mothers with detectable HBV DNA levels than mothers with undetectable HBV DNA levels [7/57 vs. 3/145, odds ratio = 5.94 (1.48–22.75)]. The risk of mother-to-infant HBV transmission increased with maternal HBV DNA levels (3/145 vs. 1/20 vs. 5/27 vs. 1/10), confirming the importance of HBV DNA in HBV mother-to-infant transmission [Wang et al., 2003; Buchanan and Tran, 2010; Ni, 2010].
In addition to HBsAg, maternal anti-HBc, anti-HBe and HBeAg can be transmitted to a fetus in utero. In the present study, 100% of the anti-HBc- or anti-HBe-positive infants had mothers who were also positive. However, the anti-HBc positivity rate remained high (21%) even after 24 months of age and lasted longer than that of anti-HBe, which is in agreement with the observations of a previous study [Wang et al., 2003]. Although one animal experiment reported that HBeAg could not efficiently cross the murine placenta [Milich et al., 1990], several clinical studies have shown that HBeAg can indeed cross the human placenta [; Zhu et al., 1997; Wang and Zhu, 2000]. In the present study, HBeAg was detected in nearly 74% of the infants at birth, consistent with previous report [Wang et al., 2003]. HBeAg disappeared in most infants during the follow-up period, except for those who became HBV carriers, indicating that HBeAg detected in infants at birth is due to transient leakage instead of HBV replication.
Limitations existed in the present study. Of the 214 enrolled infants, 31 did not attend follow-up visits and, thus, could not be analyzed. This limited the study's power to assess the incidence of intrauterine HBV transmission. However, unless the distribution of intrauterine HBV transmission was different in these infants, it would not substantially change the present findings. In this study, there was no significant difference in average maternal age at delivery, maternal educational level (which might influence the mother's behavior), or average neonatal weight between infants who attended follow-up visits and those who did not. Thus, the results obtained here are reliable. Second, the interpretation of these findings was hampered by limitations in the study design, including the small sample size and the lack of a “gold standard” (generally accepted measure) to differentiate intrauterine transmission of HBV from other mother-to-infant transmission routes. Liver biopsy is considered the gold standard for HBV diagnosis but was not used in this study for ethical reasons. Due to these limitations, a multicenter study with a larger sample size is necessary to obtain a comprehensive overview of HBV marker dynamics in this population.
In conclusion, HBV mother-to-infant transmission was observed in approximately 5% of neonates born to HBV-positive mothers. Perinatal transmission can be effectively prevented by combined administration of HBIG and HB vaccine. However, these measures do not prevent intrauterine transmission.
Acknowledgements
We thank Xiaoying Li, Jun Li, and Shaanxi Maternal and Neonatal Health Hospital for assistance with sample collection and follow-up visits.




