Association of IL28B variants with response to pegylated-interferon alpha plus ribavirin combination therapy reveals intersubgenotypic differences between genotypes 2a and 2b†
Naoya Sakamoto and Mina Nakagawa contributed equally to this work.
Abstract
Genetic polymorphisms of the interleukin 28B (IL28B) locus are associated closely with outcomes of pegylated-interferon (PEG-IFN) plus ribavirin (RBV) combination therapy. The aim of this study was to investigate the relationship between IL28B polymorphism and responses to therapy in patients infected with genotype 2. One hundred twenty-nine chronic hepatitis C patients infected with genotype 2, 77 patients with genotype 2a and 52 patients with genotype 2b, were analyzed. Clinical and laboratory parameters, including genetic variation near the IL28B gene (rs8099917), were assessed. Drug adherence was monitored in each patient. Univariate and multivariate statistical analyses of these parameters and clinical responses were carried out. Univariate analyses showed that a sustained virological response was correlated significantly with IL28B polymorphism, as well as age, white blood cell and neutrophil counts, adherence to RBV, and rapid virological response. Subgroup analysis revealed that patients infected with genotype 2b achieved significantly lower rapid virological response rates than those with genotype 2a. Patients with the IL28B-major allele showed higher virus clearance rates at each time point than those with the IL28B-minor allele, and the differences were more profound in patients infected with genotype 2b than those with genotype 2a. Furthermore, both rapid and sustained virological responses were associated significantly with IL28B alleles in patients with genotype 2b. IL28B polymorphism was predictive of PEG-IFN plus RBV combination treatment outcomes in patients infected with genotype 2 and, especially, with genotype 2b. In conclusion, IL-28B polymorphism affects responses to PEG-IFN-based treatment in difficult-to-treat HCV patients. J. Med. Virol. 83:871–878, 2011. © 2011 Wiley-Liss, Inc.
Abbreviations used:
HCV, hepatitis C virus; HCC, hepatocellular carcinoma; IFN, interferon; PEG-IFN, pegylated-interferon; RBV, ribavirin; IL28B, interleukin 28B; SNPs, single nucleotide polymorphisms; BMI, body mass index; ALT, alanine transaminase; ISDR, the interferon sensitivity determining region; ITPA, inosine triphosphatase
INTRODUCTION
Hepatitis C virus (HCV) infects around 170 million people worldwide and is characterized by a high probability of developing chronic inflammation and fibrosis of the liver, leading to end-stage liver failure and hepatocellular carcinoma (HCC) [Alter, 1997; Sakamoto and Watanabe, 2009]. Since the first report in 1986, type I interferons have been the mainstay of HCV therapy [Hoofnagle, 1994]. Current standards of care consist of a combination of ribavirin (RBV) plus pegylated interferon (PEG-IFN)-alpha for 48 weeks for infection with genotypes 1 and 4, and for 24 weeks for the other genotypes [Zeuzem et al., 2000; Fried et al., 2002]. Although this treatment improved substantially sustained virological response rates, it may result also in serious adverse effects and a considerable proportion of patients require early discontinuation of treatment. Patients of African origin have even poorer treatment outcomes [Rosen and Gretch, 1999]. Given this situation, a precise assessment of the likely treatment outcomes before the initiation of treatment may improve substantially the quality of antiviral treatment.
Recently, several studies have reported that genetic polymorphisms of the IL28B locus, which encodes interferon-λ3 (interleukin 28B), are associated with response to interferon-based treatment of chronic HCV infections with genotype 1 [Ge et al., 2009; Suppiah et al., 2009; Tanaka et al., 2009] and also spontaneous clearance of HCV [Thomas et al., 2009].
While chronic HCV infections with genotype 2 are associated with good treatment outcome, there are some refractory cases among patients infected with genotype 2, similar to genotype 1. The aims of this study were to analyze retrospectively clinical and virological factors associated with treatment response in patients with chronic HCV infection with genotype 2 who were treated with PEG-IFN plus RBV combination therapy and to clarify the relationship between IL28B polymorphism and the response to combination therapy.
PATIENTS AND METHODS
The authors analyzed retrospectively 129 patients with chronic HCV infection with genotype 2 who received combination therapy with PEG-IFN plus RBV between December 2004 and December 2009 at 10 multicenter hospitals (liver units with hepatologists) throughout Japan. All patients had chronic active hepatitis confirmed histologically or clinically and were positive for anti-HCV antibodies and serum HCV RNA by quantitative or qualitative assays. Patients with a positive test for serum hepatitis B surface antigen, coinfection with other HCV genotypes, coinfection with human immunodeficiency virus, other causes of hepatocellular injury (such as alcoholism, autoimmune hepatitis, primary biliary cirrhosis, or a history of treatment with hepatotoxic drugs), and a need for hemodialysis were excluded.
Study Design
Each patient was treated with combination therapy with PEG-IFN-α2b (Peg-Intron, Schering-Plough Nordic Biotech, Stockholm, Sweden, at a dose of 1.2–1.5 µg/kg subcutaneously once a week) or PEG-IFN-α2a (Pegasys; Roche, Basel, Switzerland, at a dose of 180 µg subcutaneously once a week) plus RBV (Rebetol, Schering-Plough Nordic Biotech or Copegus; Roche) 600–1,000 mg daily depending on the body weight (b.w.) (b.w. <60 kg: 600 mg po daily; b.w: 60–80 kg: 800 mg po daily; b.w. >80 kg: 1,000 mg po daily; in two divided doses). The duration of the combination therapy was set at a standard 24 weeks, but treatment reduction or discontinuation was permitted by doctor's decision. The rates of PEG-IFN and RBV administration achieved were calculated as percentages of actual total dose administered of a standard total dose of 24 weeks, according to body weight before therapy. During treatment, patients were assessed as outpatients at weeks 2, 4, 6, 8, and then every 4 weeks for the duration of treatment and at every 4 weeks after the end of treatment. Biochemical and hematological testing was carried out in a central laboratory. Serum HCV RNA was measured before treatment, during treatment at 4 weekly intervals, and after therapy at 4 weekly intervals for 24 weeks, by quantitative or qualitative assays.
Patient Evaluation
The following factors were analyzed to determine whether they were related to the efficacy of combination therapy: age, gender, body mass index (BMI), previous IFN therapy, grade of inflammation and stage of fibrosis on liver biopsy, pretreatment biochemical parameters, such as white blood cells, neutrophils, hemoglobin, platelet count, alanine transaminase (ALT) level, serum HCV RNA level (log IU/ml), and single nucleotide polymorphism (SNPs) in the IL28B locus (rs8099917). Liver biopsy specimens were evaluated blindly, to determine the grade of inflammation and stage of fibrosis, by an independent interpreter who was not aware of the clinical data. Activity of inflammation was graded on a scale of 0–3: A0 shows no activity, A1 shows mild activity, A2 shows moderate activity and A3 shows severe activity. Fibrosis was staged on a scale of 0–4: F0 shows no fibrosis, F1 shows moderate fibrosis, F2 shows moderate fibrosis with few septa, F3 shows severe fibrosis with numerous septa without cirrhosis and F4 shows cirrhosis.
Informed written consent was obtained from each patient who participated in the study. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and to the relevant ethical guidelines as reflected in a priori approval by the ethics committees of all the participating universities and hospitals.
SNP Genotyping
Human genomic DNA was extracted from whole blood of each patient. Genetic polymorphism of IL28B was determined by DigiTag2 assay by typing one tag SNP located within the IL28B locus, rs8099917 (22). Heterozygotes (T/G) or homozygotes (G/G) of the minor allele (G) were defined as having the IL28B minor allele, whereas homozygotes for the major allele (T/T) were defined as having the IL28B major allele.
Outcomes
The primary end point was a sustained biochemical and virological response. A sustained virological response was defined as serum HCV RNA undetectable at 24 weeks after the end of treatment. Secondary end points were a rapid virological response (HCV RNA undetectable in serum at week 4) and end-of-treatment virological response. In addition, tolerability (adverse events) and drug adherence were recorded and factors potentially associated with virological response explored.
Statistical Analysis
SPSS software package (SPSS 18J, SPSS, Chicago, IL) was used for statistical analysis. Discrete variables were evaluated by Fisher's exact probability test and distributions of continuous variables were analyzed by the Mann–Whitney U-test. Independent factors possibly affecting response to combination therapy were examined by stepwise multiple logistic-regression analysis. All P-values were calculated by two-tailed tests, and those of less than 0.05 were considered statistically significant.
RESULTS
Clinical Characteristics and Response to Therapy
The clinical characteristics and response rates to therapy of 129 patients are summarized in Tables I and II. Sixty-eight patients achieved a rapid virological response, whereas 44 patients remained HCV-RNA positive at week 4. Treatment reduction or cessation was permitted also to avoid side effects, and one patient stopped treatment at week 12 because he was anticipated to be a non-responder. On an intention-to-treat analysis, serum HCV-RNA levels were negative at the end of treatment in 125 of the 129 patients (97%) treated and, among them, 98 (76%) achieved a sustained virological response. The rapid virological response rate of patients infected with genotype 2b was lower significantly than that of patients infected with genotype 2a (P = 0.036) (Table II). The sustained virological response rate decreased with RBV drug discontinuation and dose reduction (84% and 66% with ≧80% and <80% of RBV dose, P = 0.021, Table III). Adherences to PEG-IFN did not influence a sustained virological response or end of treatment response significantly, while RBV adherence was associated significantly with a sustained virological response (Table III).
| Total number | 129 |
| Genotype (2a/2b) | 77/52 |
| IL28B SNPs (rs8099917) | |
| TT/TG/GG | 100/28/1 |
| Age (years)a | 64 (20–73) |
| Gender (male/female) | 64/65 |
| Body mass index (kg/m2)a (N = 80) | 23.7 (16.9–33.5) |
| Previous interferon therapy (no/yes) | 102/21 (unknown 6) |
| Histology at biopsy (N = 96) | |
| Grade of inflammation | |
| A0/1/2/3 | 10/53/29/4 |
| Stage of fibrosis | |
| F0/1/2/3 | 7/59/19/11 |
| White blood cells (/µl)b (N = 94) | 5,115 ± 1,630 |
| Neutrophils (/µl)b (N = 94) | 2,765 ± 1,131 |
| Hemoglobin (g/dl)b (N = 95) | 14.2 ± 1.3 |
| Platelet count (×10−3/µl)b (N = 98) | 187 ± 95 |
| ALT (IU/L)b (N = 95) | 82 ± 78 |
| Serum HCV-RNA level (log(IU/ml))a,c | 6.2 (3.6–7.4) |
| Treatment duration (>16, ≦24) | 19/110 |
- SNPs, single nucleotide polymorphisms; ALT, alanine transaminase.
- a Data are shown as median (range) values.
- b Data are expressed as mean ± SD.
- c Data are shown as log(IU/ml)).
| Character | Number/total number (%) |
|---|---|
| Overall | |
| RVR | 68/112 (61) |
| ETR | 125/129 (97) |
| SVR | 98/129 (76) |
| Genotype | 2a | 2b | P-value |
|---|---|---|---|
| RVR | 46/67 (69) | 22/45 (49) | 0.036 |
| ETR | 74/77 (96) | 51/52 (98) | NS |
| SVR | 56/77 (73) | 42/52 (81) | NS |
- RVR, rapid virological response; ETR, end of treatment response; SVR, sustained virological response.
- Bold indicated P-value of less than 0.05.
| ≧80% | <80% | P-value | |
|---|---|---|---|
| PEG-IFN adherence | |||
| ETR | 94/96 (98) | 31/33 (94) | NS |
| SVR | 75/96 (78) | 23/33 (70) | NS |
| RBV adherence | |||
| ETR | 72/73 (99) | 53/56 (95) | NS |
| SVR | 61/73 (84) | 37/56 (66) | 0.021 |
- ETR, end of treatment response; SVR, sustained virological response; PEG-IFN, pegylated interferon; RBV, ribavirin.
- The rates of PEG-IFN and RBV administration achieved were calculated as percentages of actual total dose administered of a standard total dose of 24 weeks, according to body weight before therapy.
- Bold indicated P-value of less than 0.05.
Factors Associated With a Sustained Virological Response
Next the host clinical and viral factors associated with a sustained virological response were analyzed. Univariate statistical analysis showed that six parameters were associated significantly with the sustained virological response rates, including age, white blood cells, neutrophils, adherence to RBV, rapid virological response and an IL28B SNP (rs8099917) (Table IV). There was no significant association of sustained virological response with gender, previous interferon therapy, stage of fibrosis, pretreatment HCV titer or adherence to PEG-IFN. Further multivariate analyses were conducted using significant factors identified by the univariate analysis (Table V). The multiple logistic-regression analysis showed that only a rapid virological response was associated with a sustained virological response (OR = 0.170, P = 0.019).
| SVR (n = 98) | Non-SVR (n = 31) | P-value | |
|---|---|---|---|
| Genotype (2a/2b) | 56/42 | 21/10 | |
| IL28B SNPs (rs8099917) | |||
| TT/TG + GG | 81/17 | 19/12 | 0.024 |
| Age (years)a | 56 (20–73) | 61 (40–72) | 0.002 |
| Gender (male/female) | 51/47 | 13/18 | NS |
| Body mass index (kg/m2)a | 22.8 (16.9–33.5) | 24.1 (20.3–27.6) | NS |
| Previous Interferon therapy (no/yes) | 80/14 | 22/7 | NS |
| Grade of inflammation (A0-1/2-3) | 46/28 | 15/7 | NS |
| Stage of fibrosis (F0-2/3-4) | 64/10 | 21/1 | NS |
| White blood cells (/µl)b | 5,318 ± 1,617 | 4,489 ± 1,540 | 0.032 |
| Neutrophils (/µl)b | 2,913 ± 1,139 | 2,278 ± 983 | 0.021 |
| Hemoglobin (g/dl)b | 14.2 ± 1.4 | 14.1 ± 1.1 | NS |
| Platelet count (×10−3/µl)b | 193 ± 105 | 171 ± 54 | NS |
| ALT (IU/ml)b | 79 ± 73 | 94 ± 92 | NS |
| Pretreatment Serum HCV-RNA level (log(IU/ml))a,c | 6.1 (3.6–7.4) | 6.3 (4.0–6.7) | NS |
| PEG-IFN adherence (≧80%/<80%) | 75/23 | 21/10 | NS |
| RBV adherence (≧80%/<80%) | 61/37 | 12/19 | 0.024 |
| RVR/non-RVR | 57/24 | 11/20 | 0.001 |
- SNPs, single nucleotide polymorphisms; ALT, alanine transaminase; RVR, rapid virological response.
- a Data are show as median (range) values.
- b Data are expressed as mean ± SD.
- c Data are shown as log (IU/ml)). Bold indicated P-value of less than 0.05.
| Factor | Category | Odds ratio (95% CI) | P-value |
|---|---|---|---|
| Regression analysis | |||
| RVR | RVR | 1 | 0.019 |
| Non-RVR | 0.170 (0.039–0.744) | ||
| RBV adherence | ≧80% | 1 | 0.061 |
| <80% | 0.250 (0.059–1.064) | ||
| IL28B SNPs (rs8099917) | TT | 1 | 0.104 |
| TG + GG | 0.252 (0.048–1.330) | ||
| Age | 1.087 (0.976–1.211) | 0.128 | |
| Neutrophils | 0.999 (0.997–1.001) | 0.209 | |
| White blood cells | 1.000 (0.999–1.002) | 0.504 | |
- CI, confidence interval; SNPs, single nucleotide polymorphisms; RVR, rapid virological response, RBV, ribavirin.
- Bold indicated P-value of less than 0.05.
Comparison of Sustained Virological Response Rates According to IL28B SNPs
The PEG-IFN plus RBV treatment efficacy was compared after dividing the study subjects into two groups based on IL28B alleles (Table VI). Patients homozygous for the IL28B major allele (TT allele) achieved significantly higher rapid and sustained virological response rates than those heterozygous or homozygous for the IL28B minor allele (TG/GG alleles) (P < 0.05). In addition, responses to PEG-IFN plus RBV treatment were analyzed after dividing the study subjects into those with genotype 2a and with genotype 2b. The rapid and sustained virological response rates tended to be higher in patients homozygous for the IL28B major allele than those heterozygous or homozygous for the IL28B minor allele infected with both genotype 2a and 2b, and these differences were more profound in patients infected with genotype 2b than with genotype 2a. The rapid and sustained virological response rates of patients with the major IL28B allele were higher significantly than those of patients with the minor IL28B allele infected only with genotype 2b (rapid virological response: 58% and 0% with IL28B major and hetero/minor, P = 0.002, sustained virological response: 88% and 44% with IL28B major and hetero/minor, P = 0.009).
| Character | IL28B major | IL28B hetero/minor | P-value |
|---|---|---|---|
| Number/total number (%) | |||
| Overall | |||
| RVR | 58/88 (66) | 10/24 (42) | 0.031 |
| SVR | 81/100 (81) | 17/29 (59) | 0.013 |
| Genotype 2a | |||
| RVR | 36/50 (72) | 10/17 (59) | NS |
| SVR | 43/57 (75) | 13/20 (65) | NS |
| Genotype 2b | |||
| RVR | 22/38 (58) | 0/7 (0) | 0.002 |
| SVR | 38/43 (88) | 4/9 (44) | 0.009 |
- RVR, rapid virological response; ETR, end of treatment response; SVR, sustained virological response.
Although the rapid virological response rate of patients infected with genotype 2b was lower significantly than that of patients infected with genotype 2a, the sustained virological response rate was higher in patients infected with genotype 2b than with genotype 2a (Table II), In order to investigate that discrepancy, sustained virological response rates in patients with or without rapid virological response were analyzed according to IL28B SNPs. In patients infected with genotype 2b and a non-rapid virological response, the sustained virological response rates differed significantly between IL28B major and hetero/minor groups (sustained virological response with non-rapid virological response: 75% and 29% with IL28B major and hetero/minor, P = 0.044), and no one achieved a rapid virological response among the patients infected with genotype 2b and with the IL28B hetero/minor allele. In patients infected with genotype 2a, on the contrary, there was no significant correlation of rapid and sustained virological response rates between IL28B SNPs (sustained virological response with rapid virological response: 78% and 70% with IL28B major and hetero/minor, P = 0.630, sustained virological response with non-rapid virological response: 57% and 43% with IL28B major and hetero/minor, P = 0.552).
Next, changes in virological response rates over time were investigated in patients treated with PEG-IFN plus RBV and the time course was analyzed after separating the patients infected with genotype 2a and 2b (Fig. 1). Patients with IL28B-TG and -GG showed significantly lower rates of rapid and sustained virological response, compared to patients with IL28B-TT, and greater differences were observed according to IL28B SNPs among patients infected with genotype 2b than with 2a.
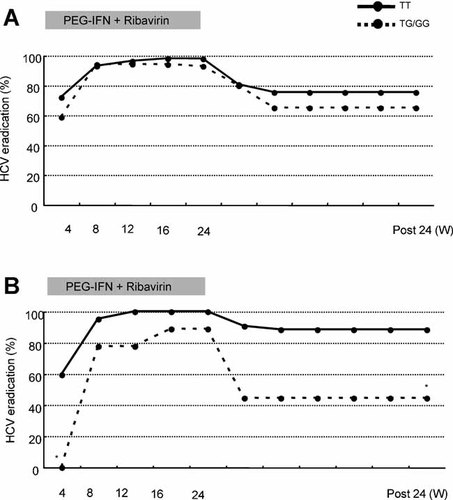
Changes over time in virological response rates were confirmed in patients treated with PEG-IFN plus RBV, and the time courses were analyzed after separating the patients infected with genotypes 2a and 2b. Patients with the IL28B major (TT allele) are indicated in the figure by a continuous line and those with IL28B hetero or minor (TG or GG), by a dotted line. IL28B-TG and -GG patients showed significantly lower rates of rapid and sustained virological response, compared to IL28B-TT patients. P-values were two-tailed and those of less than 0.05 were considered to be statistically significant. *P < 0.01.
Side Effects
Side effects leading to Peg-IFN plus RBV discontinuation occurred in eight patients (6.2%) and discontinuation of RBV alone occurred in four patients (3.1%). Among the eight patients who withdrew from both drugs, four, including one who stopped at week 7, had achieved a sustained virological response. Among four patients who withdrew from RBV alone, three had achieved a sustained virological response. The events leading to drug withdrawal were HCC treatment (n = 2), general fatigue (n = 2), retinopathy, neuro-psychiatric event, severe dermatological symptoms suggestive of the drug-induced hypersensitivity syndrome, and arrhythmia.
DISCUSSION
Recent studies suggest that genetic variations in IL28B are strongly associated with response to therapy of chronic HCV infection with genotype 1 [Ge et al., 2009; Suppiah et al., 2009; Tanaka et al., 2009] and with spontaneous HCV clearance [Thomas et al., 2009]. In this study, univariate analyses showed that the sustained virological response was correlated significantly with IL28B polymorphism (rs8099917) as well as age, adherence to RBV and rapid virological response, and multiple logistic-regression analysis showed that only a rapid virological response was associated with a sustained virological response in all patients infected with genotype 2 (Table V). Although the IL28B polymorphisms are not so useful for predicting the clinical outcomes of PEG-IFN plus RBV combination therapy among patients with genotype 2, compared to genotype 1, IL28B polymorphism was predictive of PEG-IFN plus RBV treatment outcomes among patients with genotype 2 and, more remarkably, among patients with genotype 2b in this study. Indeed, both rapid and sustained virological response rates according to the rs8099917 genotypes were different significantly in patients with genotype 2b but not in patients with genotype 2a. Furthermore, in the plot of virological response (Fig. 1), a stronger effect of the IL28B allele was observed in patients with genotype 2b than with genotype 2a.
It has been reported that there was no significant association between genetic variation in IL28B and response to therapy of HCV patients infected with genotype 2 or 3, indicating that the prognostic value of the risk allele for treatment response might be limited to individuals with difficult-to-treat HCV genotypes [Rauch et al., 2010]. This report lacks details of the distribution of the various genotypes. The present study agrees with a more recent report that the IL28B polymorphism was associated with a sustained virological response in patients with chronic HCV infection with genotype 2 or 3 who did not achieve a rapid virological response [Mangia et al., 2010]. In Japan, the percentage of HCV infection with genotype 1b is 70%, genotype 2a is 20% and genotype 2b is 10%, whilst other genotypes are observed only rarely. In this study, the association of IL28B polymorphism with response to therapy was analyzed in more detail, considering the subtypes 2a and 2b, and IL28B polymorphism (rs8099917) found to be linked more closely to the virological response of patients infected with genotype 2b than those with genotype 2a. A recent in vitro study, which constructed several chimeric virus clones between HCV-2b and HCV-JFH1 (2a), also supported subgenotypic differences between genotype 2a and 2b [Suda et al., 2010]. The authors speculated that the prognostic value of the risk allele for treatment response might be more pronounced in individuals with difficult-to-treat HCV subgenotypes, such as patients infected with genotype 2b, compared with 2a. In addition, the prevalence of the IL28B minor allele is much higher in Caucasians and African Americans than in eastern Asian populations [Thomas et al., 2009], which suggest that the effects of IL28B polymorphism could be more pronounced in non-Asian populations. In the present results, however, the sustained virological response rate of patients infected with genotype 2b was higher than that of patients with genotype 2a overall. We speculate that, among patients infected with genotype 2b, only those with the IL28B minor variant might be treatment-refractory. That possibility might be validated further by a larger cohort study with genotype 2b.
The sustained virological response rates decreased significantly with failure of adherence to RBV (Table III), which was extracted as a factor associated with sustained virological response by univariate analysis (Table IV). Regardless of the drug adherence, end of treatment response rates of patients infected with genotype 2 were around 94–99%, but the sustained virological response rates of the patients who received a total cumulative treatment dose of RBV of <80% was reduced significantly. As reported previously, increased RBV exposure during the treatment phase was associated with an increased likelihood of a sustained virological response [McHutchison et al., 2009] and these results confirm the importance of RBV in order to prevent relapse. Furthermore, host genetic variation leading to inosine triphosphatase (ITPA) deficiency protects against hemolytic anemia in chronic hepatitis C patients receiving RBV as revealed recently [Fellay et al., 2010]. We have reported also that the ITPA SNP, rs1127354, is confirmed to be a useful predictor of RBV-induced anemia in Japanese patients and that the incidence of early dose reduction was significantly higher in patients with ITPA-major (CC) variant as expected and, more importantly, that a significant higher sustained virological response rate was achieved in patients with the ITPA-hetero/minor (CA/AA) variant with non-genotype 1 or low viral loads [Sakamoto et al., 2010].
A rapid virological response was extracted in this study as a factor associated with sustained virological response only by multivariate analysis. It has been reported recently that a rapid virological response is an important treatment predictor and that drug adherence, which is reported to affect the therapeutic efficacy in patients infected with genotype 1, had no impact on the both sustained and rapid virological responses in combination therapy for patients infected with genotype 2 [Inoue et al., 2010]. The reasons why several host factors useful for predicting the response to therapy in patients with genotype 1, such as gender, age, progression of liver fibrosis and IL28B polymorphism had no influence on the efficacy in patients with genotype 2, can be attributed to IFN-sensitive genotypes. Similarly, the other viral factors useful for predicting the response to therapy, such as viral load and amino acid substitutions in the Core and NS5A regions had no influence on treatment outcomes. In this study, patients who achieved a rapid virological response had a high sustained virological response rate, regardless of IL28B polymorphism in patients with genotype 2a but, interestingly, none of the IL28B-TG and -GG patients with genotype 2b achieved a sustained virological response (although there were nine IL28B-TG and -GG patients with genotype 2b, two could not be determined as rapid virological response because the times at which they became HCV-negative were not recorded clearly, being described as 4–8 weeks.) These results also suggest that patients with both genotype 2b and IL28B minor allele are refractory cases.
IL28B encodes a protein also known as IFN-λ3 [O'Brien, 2009]. IL28A (IFN-λ2) and IL29 (IFN-λ1) are found adjacent to IL28B on chromosome 19. These three IFN-λ cytokines, discovered in 2003 by two independent groups [Kotenko et al., 2003; Sheppard et al., 2003] have been suggested to be involved in the suppression of replication of a number of viruses, including HCV [Robek et al., 2005; Marcello et al., 2006; Tanaka et al., 2010]. Humans have these three genes for IFN-λ, and this group of cytokines is now collectively referred to as type III IFN [Zhou et al., 2007]. IFN-λ functionally resembles type I IFN, inducing antiviral protection in vitro [Kotenko et al., 2003; Sheppard et al., 2003] as well as in vivo [Ank et al., 2006]. Type III IFN utilizes a receptor complex different from that of type I IFN, but both types of IFN induce STAT1, STAT2, and STAT3 activation by activation of a highly overlapping set of transcription factors, and the two types of IFN seem to have similar biological effects at a cellular level. Some in vitro studies have suggested that IFN-α induces expression of IFN-λ genes [Siren et al., 2005]. Other in vitro studies also suggest that IFN-λ inhibits hepatitis C virus replication through a pattern of signal transduction and regulation of interferon-stimulated genes that is distinct from IFN-α and that the anti-HCV activity of either IFN-α or IFN-λ is enhanced by a low dose of the other [Marcello et al., 2006]. A novel mechanism of the interaction between IFN-α and IFN-λ may play a key role in the suppression of HCV [O'Brien, 2009].
In conclusion, IL28B polymorphism is predictive of PEG-IFN plus RBV treatment outcomes in patients infected with genotype 2, and more remarkably with genotype 2b. These results suggest that IL-28B polymorphism affects responses to IFN-based treatment in more difficult-to-treat subpopulations of HCV patients, and that intersubgenotypic differences between genotype 2a and 2b are revealed by responses to PEG-IFN plus RBV treatment according to IL28B variants.
Acknowledgements
The study is based on 10 multicenter hospitals throughout Japan, in the Kanto area (Tokyo Medical and Dental University Hospital, Musashino Red Cross Hospital, Kashiwa City Hospital, Kudanzaka Hospital, Showa General Hospital, Tsuchiura Kyodo General Hospital, Toride Kyodo General Hospital), Tokai area (Nagoya City University Hospital, Mishima Social Insurance Hospital) and Chugoku/Shikoku area (Ehime University Hospital).




