Evaluation of type-specific HPV persistence and high-risk HPV viral load quantitation in HPV positive women under 30 with normal cervical cytology
Abstract
The persistence of high-risk HPV (HR-HPV) infection is necessary for the development of cervical intraepithelial neoplasia. The aim of this study was to evaluate if HR-HPV typing and HPV16, 18, 31, and 33 quantitation are predictive for type-specific infection persistence and/or the development of CIN in women under 30 with normal cervical cytology. Young women (under 30) attending a family planning clinic who were HPV positive with normal cervical cytology were included. HPV genotyping was assessed by MY09/MY11 PCR, sequencing, phylogenetic analysis, and cloning when necessary. HR-HPV viral load was quantified using duplex real-time PCR. Study patients were offered for a second smear and HR-HPV detection and quantitation after 12 months. HR-HPV was identified in 43 (21.9%) of the 199 included women. Of these, 39 patients had a second cervical sample taken within a mean interval of 11.7 months (8.8–18.3 months). The mean HR-HPV 16, 18, 31, and 33 initial viral load was 1.9 × 106 copies/million cells. The level of viral load did not reveal any significant association with type-specific HR-HPV persistence or the subsequent development of cervical intraepithelial neoplasia. Only HPV16 infection was significantly more likely to persist (91.7% vs. 33.1%, P = 0.001) and to develop CIN (33.3% vs. 3.7%, P = 0.025). In women under 30 with normal cytology, HR-HPV viral load is common and is not predictive of HPV persistence or the development of cervical intraepithelial neoplasia. HPV16 positive women are significantly more likely to have persistent infection and to develop cervical intraepithelial neoplasia. J. Med. Virol. 83:637–643, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
High-risk or oncogenic, human papillomavirus (HR-HPV) infection is the major risk factor for the development of cervical cancer and cervical intraepithelial neoplasia. In France, 97% of cervical cancers are HPV positive [Pretet et al., 2007]. Among HR-HPV types, HPV16, is by far the most prevalent type and is identified in 73% of cervical cancers, followed by HPV 18 (19%), 31 (7%), and 33 (4%) [Pretet et al., 2007]. For this reason HPV testing has been considered as an alternative method of cervical pre-cancer screening. Compared to cervical cytology, HPV testing is more sensitive but less specific in the detection of grade 2 or more cervical intraepithelial neoplasia [Arbyn et al., 2006; Cuzick et al., 2006; Koliopoulos et al., 2007]. The Superiority of HPV testing over cytology or visual inspection techniques has also been demonstrated in low resource setting countries [Sankaranarayanan et al., 2009]. In this study screening with HPV and subsequent management of screen positive women has been shown to result clinically important and statistically significant reduction in the number of advanced stages cervical cancers and of overall cervical cancer related mortality.
Age has a major impact on the clinical utility of HPV testing being very much more specific in women over 30 years [Koliopoulos et al., 2007; Mayrand et al., 2007]. HPV infection is extremely common in young women and in the great majority of cases it is both transient and ultimately harmless. Following initial exposure to HR-HPV, the great majority of women will clear their infection in 8–10 months without the development of a cervical lesion [Ho et al., 1998; Woodman et al., 2001; Dalstein et al., 2003]. Thus, persistence of HPV infection only affects a small minority of women [Ho et al., 1998; Franco et al., 1999; Bosch and Munoz, 2002]. More than HR-HPV infection itself, it is the persistence of the same HR-HPV infection that has been demonstrated to be the most important risk factor for cervical intraepithelial neoplasia [Nobbenhuis et al., 1999; Schlecht et al., 2001; Dalstein et al., 2003]. Thus, the positive predictive value of a single HPV test in young women is poor and only a minority of these women will develop a cervical lesion [Cuzick et al., 2003]. Factors that influence HR-HPV persistence remain unknown and predictive factors for HR-HPV infection persistence would be clinically valuable.
With the increased use of HPV testing in routine practice, physicians must consider how to manage HPV positive young women who have normal cytology. This presents a clinical dilemma and may easily result in unnecessary treatment with the potential for subsequent pregnancy related morbidity and perinatal mortality [Kyrgiou et al., 2006; Arbyn et al., 2008]. Determination of HR-HPV viral load would be useful if it could predict HR-HPV persistence and Cervical Intraepithelial Neoplasia [van Duin et al., 2002; Dalstein et al., 2003; Lai et al., 2008; Bae et al., 2009; Munoz et al., 2009]. The predictive value of HR-HPV detection could be HPV type-dependant with perhaps a higher risk for women with high HPV 16 viral load [Moberg et al., 2004, 2005; Gravitt et al., 2007].
The aim of this study was to evaluate HPV type-specific prevalence and persistence in a population of women under 30 with normal cervical cytology. In that specific population, HPV 16, 18, 31, and 33 viral load, referred to as “oncogenic” HPV viral load, was evaluated as a predictive factor of type-specific HPV infection persistence and of colposcopically documented cervical intraepithelial neoplasia. The impact of HPV genotyping was also evaluated.
MATERIALS AND METHODS
Patients and Design of the Study
The study design is summarized in Figure 1. All women under 30 with no previous history of cervical lesion or of abnormal cervical cytology attending an Irish Family Planning Association (IFPA) clinic in Tallaght (Dublin, Ireland) for routine screening cervical cytology were invited to participate to the study. All had a liquid-based cervical cytology performed using the ThinPrep® pap test (Hologic, Inc., Bedford, MA) and dedicated cervical brush. Women with abnormal cervical cytology were referred for colposcopic examination or repeat cervical cytology according to British Society of Colposcopy and Cervical Pathology (BSCCP) guidelines and were then excluded form the study. All women with normal cervical cytology had a HPV test performed directly on the remaining suspension of the ThinPrep® pap test vial. HPV negative women were excluded from the study and referred for routine screening cytology, that is, three yearly. HPV positive women were asked to return in 1-year for a repeat cervical cytology and HPV test. Subsequent cervical smears were performed at the Colposcopy Clinic of the Coombe Women and Infants University Hospital. All were tested for HPV with systematic genotyping of HPV positive sample and quantitation of HPV 16, 18, 31, and 33 viral load. Women with a second abnormal smear were referred for colposcopic examination. Colposcopic examinations were blinded to the results of HPV genotyping and viral load quantitation.
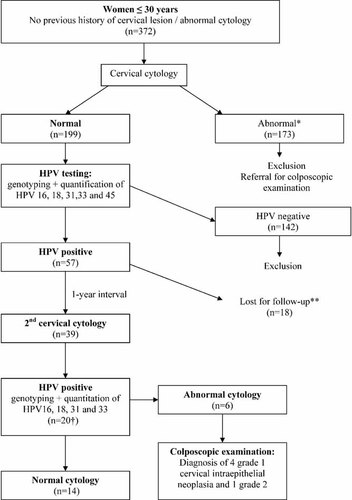
Study design. *Smear was considered abnormal if borderline nuclear abnormalities (BNA) or more severe abnormalities were identified. **One patient got pregnant and thus did not attend to the 2nd cervical smear. †Twenty cases of type-specific HPV persistent infection were identified: 11 HPV16, 4 HPV 18, 1 HPV 31, 0 HPV 33, 3 LR-HPV, and 1 HR-HPV different from types 16, 18, 31, and 33.
Study protocol received approval from Ethics Committees of both the Coombe Women and Infants University Hospital and the Adelaïde and Meath Hospital in Tallaght. Informed consent was obtained from all the women participating to the study.
Cervical Cytology Analysis
All cervical smears were analyzed at the Pathology department of the Coombe Women and Infants University Hospital (Dublin, Ireland). Analysis were performed using ThinPrep® Imaging system (Hologic, Inc.). All slides were first screened using the Review Scopes® followed by manual secondary screening. Negative cases were signed out by the cytotechnologists and all abnormal cases (Borderline Nuclear Abnormality or greater) were signed out by the consultant Pathologist. Cervical cytology analysis were blinded to the result of HPV genotyping and viral load quantitation.
Virological Analysis
HPV testing was performed at the department of Virology of the Timone's Hospital (Marseilles, France). All HPV tests were performed using the remaining fluid in the liquid based cytology vials. HPV genotyping was assessed by PCR (MY09/MY11 primers), sequencing, phylogenetic analysis and cloning if necessary, as described previously [Tamalet et al., 2010]. Quantitation of HPV 16, 18, 31, and 33 positive samples were performed using quantitative duplex real-time PCR method, as reported previously [Carcopino et al., 2006]. HPV viral load was expressed as the number of HPV copies/million cells.
For quantification, we used a plasmid which contained the five target sequences of interest: HPV 16, HPV 31, HPV 33, HPV 45 (each on E6 gene), HPV 18 (on E7 gene), and human albumin gene (on exon 12). This method allowed HPV 16 and 18, 31, 33, and 45 and albumin gene copy number to be quantified in the same assay. So, HPV viral load could be expressed as HPV copies per cell or per million cells. For the purpose of intra-assay reproducibility, the standard curve was tested 10 times on the same plate and serial dilutions of positive samples (from 107 copies to 1 copy per assay) each with a high HPV 16, 18, 31, 33, or 45 value were tested in duplicate to establish the sensitivity and the reproducibility of the method. We obtained a lower detection limit of 5 copies per 5 µl of sample as well for HPV 16,18, 31, 33, 45 as for albumin. The linearity was excellent from the highest value to 1 copy per 5 µl of sample. The data revealed an excellent reproducibilirty of the assays as well for high, median, or low values. The coefficient of correlation varied from 98.9% for HPV 18 to 99.7% for HPV 16. For the purpose of inter-assay reproducibility, the coefficient of variation (CV) was determined for each reaction. Mean CV for each point of the quantification curve in HPV 16, 18, 31, 33, 45, and albumin was <3%.
Statistical Analysis
Statistical analysis was performed using SPSS 15.0 software for Windows (SPSS, Inc., Chicago, IL). Groups were compared using the Chi square test or the Fisher exact test for qualitative characteristics, and using the Mann–Whitney test for continuous ones. A two-sided P-value of less than 0.05 was considered statistically significant.
RESULTS
Recruitment ran from September 2007 to the end of March 2009, during which 372 patients participated in the study. Of these, 199 had normal cytology. One hundred seventy three had an abnormal cervical smear, were excluded from the study and were referred for colposcopic examination or repeat cervical cytology. All of the 199 patients whose cervical cytology was normal were tested for HPV. The overall HPV prevalence was 28.6% (57/199) (Table I). HR-HPV was identified in 43 (21.6%) patients. The most prevalent HPV genotypes were HPV 16 (11.1%) and HPV 18 (5.5%) followed by HPV 33 (4%), HPV 6 (4%), HPV 31 (3%), HPV 53, 58, 66, 61, 62, and 83 (3.1%). Single HPV infection was observed in 41 (20.6%) while 16 (8%) patients had at least two different HPV types. The mean HR-HPV viral load for HPV 16, 18, 31, and 33 was 1.5 × 106 copies/million cells (Standard Deviation (SD): 3.05 × 106). The mean HPV 31 viral load was statistically higher than the mean HPV 16 (P = 0.015) or HPV 18 viral loads (P = 0.019; Fig. 2).
| HPV test | ||
|---|---|---|
| Initial (n = 199) | 1-year follow up (n = 39) | |
| Number of HPV types detected n (%) | ||
| 1 | 41 (20.6) | 17 (43.6) |
| ≥2 | 16 (8) | 8 (20.5) |
| HPV types detected n (%) | ||
| HPV16 | 22 (11.1) | 15 (38.5) |
| HPV18 | 11 (5.5) | 8 (20.5) |
| HPV31 | 6 (3) | 2 (5.1) |
| HPV33 | 8 (4) | 0 (0) |
| Other HR-HPV typesa | 5 (2.5) | 1 (2.6) |
| LR-HPV typesb | 25 (12.6) | 7 (17.9) |
| HPV viral load (n copies/106 cells) mean (SD) | ||
| HPV16 | 8.5 × 105 (2.04 × 106) | 5.27 × 105 (1.08 × 106) |
| HPV18 | 9.4 × 105 (2.87 × 106) | 3.66 × 105 (9.24 × 105) |
| HPV31 | 4.57 × 106 (4.65 × 106) | 2.11 × 108 (2.88 × 108) |
| HPV33 | 2.37 × 106 (3.85 × 106) | — |
- HR-HPV, high-risk HPV; LR-HPV, low-risk HPV.
- a Other high-risk HPV types initially detected: 4 (2%) HPV58 and 1 (0.5%) HPV82.
- b Low-risk HPV types initially detected—25 patients were infected by 30 LR-HPV types (because of multiple infections): 8 (4%) HPV6, 6 (3%) HPV53, 4 (2%) HPV66, 2 (1%) HPV61, 2 (1%) HPV62, 2 (1%) HPV 83, 1 (0.5%) HPV1, 1 (0.5%) HPV11, 1 (0.5%) HPV54, 1 (0.5%) HPV70, 1 (0.5%) HPV81, and 1 (0.5%) HPV84.
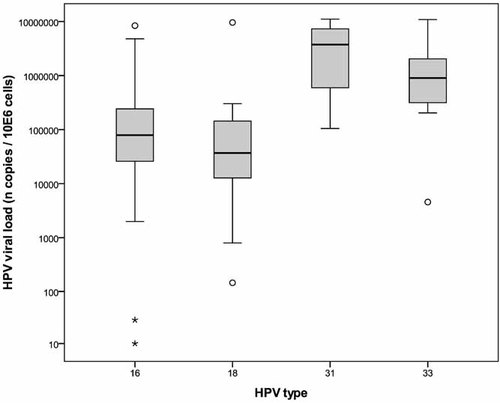
Distribution of HPV16, 18, 31, and 33 viral load in women under 30 with normal smear at the time of inclusion (logarithmic scale). HPV31 viral load was statistically higher than HPV16 (P = 0.015) and HPV18 viral load (P = 0.019).
After the exclusion of all HPV negative women, 57 remained for analysis. Of these, 39 (68.4%) patients had a second cervical sample taken within a mean interval of 11.7 months (min–max: 8.8–18.3 months). One patient conceived and declined a second cervical sample. Seventeen patients were lost to follow up despite several reminder invitations by mail and telephone (Fig. 1). The mean patient age was 24.8 years (min–max: 18–30 years). The mean interval between smears was comparable for HPV 16, 18, 31, and 33 positive patients as well as for patients who were positive for other HPV types.
All of the 39 patients were tested for HPV. Type-specific persistent HPV infection was identified in 20 (51.3%). The mean age of patients with persistent infection and of those who cleared their infection was comparable: 24.7 versus 24.5 years, respectively. HPV 16 was significantly more likely to persist than were all other types: 11 (91.7%) versus 9 (33.3%), respectively (P < 0.001).
Compared to HR-HPV types, low-risk HPV (LR-HPV) were significantly less likely to persist: 17 (54.8%) versus 3 (15.0%), respectively (P = 0.004; Table II). In HPV 16, 18, 31, and 33 positive patients the initial overall viral load was not predictive of type specific HPV infection. Mean initial HPV viral load of women with persistent HPV 16, 18, 31, or 33 infection was 1.2 × 106 copies/million cells whereas viral load of women who had cleared their infection was 5.3 × 106 copies/million cells (P = 0.173).
| HPV type initially detected | Number of patients followed-up | 1-year specific persistent HPV infection rate (%) [95% CI] | Cervical intraepithelial neoplasia | |||
|---|---|---|---|---|---|---|
| All grades | Grade 2 or 3 | |||||
| Incidence rate (%) [95% CI] | Women with 1-year specific persistent HPV infection, n (%) | Incidence rate (%) [95% CI] | Women with 1-year specific persistent HPV infection, n (%) | |||
| HPV16 | 12 | 92 [62-100] | 33 [10–65] c | 3 (75) | 8 [0–39] | 1 (100) |
| HPV18 | 7 | 57 [18–90] | 14 [0–58] | 0 (0) | 0 | — |
| HPV31 | 5 | 20 [1–72] | 0 | — | 0 | — |
| HPV33 | 3 | 0 | 0 | — | 0 | — |
| Other HR-HPV typesa | 4 | 25 [1–81] | 0 | — | 0 | — |
| LR-HPV typesb | 20 | 15 [3–38] | 5 [1–25]d | 0 (0) | 0 | — |
- HR-HPV, high-risk HPV; LR-HPV, low-risk HPV.
- a Other high risk HPV types: 3 HPV58 and 1 HPV82.
- b Low risk HPV types: 6 HPV53, 4 HPV6, 2 HPV62, 2 HPV66, 2 HPV83, 1 HPV11, 1 HPV61, 1 HPV 81, and 1 HPV84.
- c Three patients were diagnosed with grade 1 Cervical intraepithelial neoplasia and one with grade 2 cervical intraepithelial neoplasia.
- d One patient was diagnosed with grade 1 cervical intraepithelial neoplasia. Bold style indicates statistical significance when comparing with other HPV types.
The result of a second smear test was abnormal in 6 of the 39 patients. Three women were reported as having borderline nuclear abnormality (BNA), 2 low-grade squamous intraepithelial lesion (LSIL) and 1 high-grade squamous intraepithelial lesion (HSIL). After colposcopic examination, one patient was diagnosed with normal cervix. Grade 1 cervical intraepithelial neoplasia was diagnosed in four patients who were advised to have a follow up colposcopy and smear within a year. One patient was diagnosed with grade 2 cervical intraepithelial neoplasia, confirmed at large loop excision of the transformation zone (LLETZ).
Patients diagnosed with cervical intraepithelial neoplasia (any grade) were not significantly more likely to have persistent HPV infection: 3 (60%) versus 17 (50%), respectively, though these numbers do not allow accurate assessment of risk. Nor was the initial HPV 16, 18, 31, or 33 viral load predictive of cervical intraepithelial neoplasia. Initial mean HPV viral load in patients who were diagnosed with cervical intraepithelial neoplasia was 5.2 × 104 copies/million cells compared to 2.9 × 106 copies/million cells in women with a normal cervix (P = 0.263). Also the mean age was comparable in both groups: 23.7 versus 24.7 years (P = 0.515). Finally, compared to other HPV types, only HPV 16 infection was significantly associated with a higher probability of developing cervical intraepithelial neoplasia: 1 (3.7%) versus 4 (33.3%), respectively (P = 0.025; Table II). Despite the fact that the only patient diagnosed with grade 2 cervical intraepithelial neoplasia was HPV 16 positive, HPV 16 infection was not significantly associated with higher probability of Grade 2 or 3 cervical intraepithelial neoplasia: 1 (8.33%) versus 0 (0%), respectively (P = 0.3).
DISCUSSION
With a HPV and HR-HPV prevalence of 28.6% and 21.6%, respectively, the present study confirms that HPV, and particularly HR-HPV infection, is very common in women under 30 years old with normal cervical cytology. Such finding concurs with the results of other studies. In women who reported recent coitarche and who reported just one sexual partner, the 1-year cumulative incidence rate of HPV infection is 28.5% (IC at 95%: 20.6–38.6%) and reaches almost 50% after 3 years [Winer et al., 2008]. The overall cumulative incidence rate of HPV infection in 25 years old women has been estimated to be 80% [Winer et al., 2006]. Thus, the prevalence of HPV infection in young women is very high, with a peak of prevalence of 19.6% for women between 25 and 29 years. This appears to decline to 4.3% in women over 30 [Jacobs et al., 2000]. Although the rates for HR-HPV are generally lower, the prevalence and natural history is comparable to overall HPV. The best evidence suggests that in 20–24 and 25–29 years old women, HR-HPV prevalence is 13% and 17% respectively and will decrease to 2.5% and 3.9% in women over 30 [Jacobs et al., 2000; Cibas et al., 2007]. Interestingly, despite the limited number of 39 women in the key core study group, it should be noted that 20 women (51.3%) presented persistent HR-HPV infection. Of these 20 women, six had abnormal cytology, while 14 had sustained normal cytology after a mean interval time period of 11.7 months. Thus, since the critical point is actually to know the future of this persistent HPV infection in these 14 young women it will be of highest importance to monitor their cytological and virological follow up. What is more, given the consistent association between persistent infection and increased risk of cervical precancer and cancer, persistent HPV infection was the virological endpoint proposed as a relevant clinical marker in the Cervarix vaccine trial [Paavonen et al., 2009]. Finally the high incidence of transient HPV infection in young women could also explain the higher probability of abnormal smear in young women as the cytopathic effects of HPV can be detected on cytology [ACOG, 2009]. However, explanation for higher probability of abnormal cytology, as observed in the present study, is probably multifactorial and includes the low socioeconomic level of women attending to the IFPA clinic in Tallaght [Cotton et al., 2007].
In the present study the initial HPV 16, 18, 31, and 33 viral load was not found to be predictive of type-specific HPV persistence or the development of cervical intraepithelial neoplasia although in other studies HPV viral load has been demonstrated to be correlated with HPV persistence and subsequent cervical pre-malignancy [van Duin et al., 2002; Dalstein et al., 2003; Lai et al., 2008; Bae et al., 2009; Munoz et al., 2009]. This correlation may vary with HPV type and to be most reliable for HPV 16 [Moberg et al., 2004, 2005; Gravitt et al., 2007]. It seems that no previous reports have evaluated such a correlation in young women with normal smears.
Interestingly, mean HR-HPV viral load was relatively high. Indeed, the mean initial HR-HPV viral load measured in the present study was close to the HPV 16 viral load cut-off (>2.2 × 107 copies/million cells) identified by Saunier et al. [2008] to allow the identification of women with prevalent grade 2 or 3 cervical intraepithelial neoplasia with a high specificity. Thus, regardless of age, it has been shown that HR-HPV viral load increases significantly with the severity of cervical lesions [Saunier et al., 2008]. But, in a previous study, such significant association was only found in patients over 30 while mean HPV 16 and 18 viral load was already particularly high in women under 30 [Carcopino et al., 2006]. These results suggest that HR-HPV viral load is commonly high in young women, possibly related to the recent occurrence of HPV infection, and would therefore not be of any clinical significance.
In this study, only HPV 16 was found to persist and associated with an increased risk of cervical intraepithelial neoplasia. In comparison, LR-HPV types were significantly less likely to persist. This finding illustrates the high oncogenicity of HPV 16 and points to the potential benefit of HPV genotyping in clinical practice. Indeed, HPV 16 has been already shown to persist over a 8–16 months period [Ralston Howe et al., 2009]. The usefulness of HPV 16 testing in the management of HPV positive women with normal cervical cytology has been already demonstrated [Khan et al., 2005]. In their study the detection of HPV 16 was associated with a 17.2% risk of grade 3 or more cervical intraepithelial neoplasia over a 10 years period (95% CI: 11.5–22.9). In the present study, the absence of significant association between HPV 16 infection and grade 2 or more cervical intraepithelial neoplasia occurrence may be explained by the small number of HPV positive patients and because the study was limited to 1-year's follow-up.
The Management of HPV positive young women with normal cytology remains a clinical challenge. The balance between not overtreating women with a minimal risk of progression to cancer and the risk of treatment related morbidity is a fine line [Kyrgiou et al., 2006; Arbyn et al., 2008]. These results suggest that the use of HPV genotyping and particularly HPV 16 identification may be valuable. Because of the risk of HPV 16 persistence and the development of cervical intraepithelial neoplasia, young HPV 16 positive patients with normal smear should be monitored carefully.
CONCLUSION
This study extends the understanding of the natural history of HPV in women under 30 with normal cervical cytology. In this specific population, HPV type 16, 18, 31, and 33 viral load is often relatively high. It does not appear to predict type-specific HPV infection persistence or the development of cervical intraepithelial neoplasia. However, in the present study, HPV 16 infection was more likely to persist and HPV 16 positive patients were also more likely to develop cervical intraepithelial neoplasia. The use of HPV typing may be valuable in women under 30.
Acknowledgements
We are particularly grateful to the Tallaght's Irish Family Planning Association (IFPA) and particularly to Annet Smith for their great participation to this study. We also want to thank all the patients who accepted to be part of this study. Finally, we thank Fidelma Kavanagh and all the staff of the colposcopy clinic of the Coombe Women's Hospital for their help on the management of patients and samples.




