Seroprevalence and serum profile of cytomegalovirus infection among patients with hematologic disorders in Bahia State, Brazil
Abstract
Human cytomegalovirus (CMV) is a ubiquitous herpesvirus with from 30% to 100% of the general population exhibiting prior exposure by serology. This cross-sectional study evaluated the serological profile of anti-CMV antibodies and two acute-phase reaction proteins in Haematologic Disorder Patients (HDPs) from Bahia State, Brazil. Immuno-chemiluminescence assays were performed to detect anti-CMV IgM and IgG antibodies. Serological levels of High Sensitivity C-Reactive Protein (CRPH) and Alpha-1-Acid Glycoprotein (AAG) were measured using immunonephelometry. A total of 470 HDPs were enrolled, 238 (50.6%) males and 232 (49.4%) females. The overall seroprevalence of CMV was 89.4%, directly proportional to age and to the amount of blood units transfused. There was no difference between seroprevalence rates according to gender (P = 0.12). Four HDPs (0.9%) were seropositives for anti-CMV IgM, only one could be characterized as recent acute infection. The most CMV seropositive HDPs had anti-CMV IgG in low titers. There was a tendency for females to have higher anti-CMV IgG titers than men (P < 0.05). CRPH levels were different among HDPs CMV negative and positive groups (P < 0.001). There was no difference in the AAG levels between groups (P = 0.15). The high CMV seroprevalence found underscores the importance of using strategies to provide “CMV safe” blood to HDPs who are at high risk of developing severe CMV infection. CRPH can be used as a biomarker associated with CMV seropositivity; however, more efforts are needed to better characterize the clinical profile of active CMV infection in this group of patients. J. Med. Virol. 83:298–304, 2011. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Human cytomegalovirus (CMV) is a ubiquitous type 5 beta human herpesvirus (HHV-5), with seroprevalence ranging between 30% and 100% that is directly proportional to age and inversely proportional to socioeconomic status [Staras et al., 2006; Junqueira et al., 2008; Crough and Khanna, 2009].
The potential of CMV to be transmitted through many different means (including interaction with infected bodily fluids, organ transplants, congenital or perinatal infection, and blood transfusion) contributes greatly to its widespread dissemination. Some individuals are considered at “high-risk” for developing severe CMV infections, such as infants with low birth weights, transplant recipients, HIV positives, cancer patients, or even those immunosuppressed by medication [Crough and Khanna, 2009; Capria et al., 2010]. Controlling infections remains a challenge both for treatment units and for hemotherapy services [Fontaine et al., 2010].
It is estimated that the risk of transfusion transmitted-CMV (TT-CMV) to CMV seronegative patients ranges from 2.7% to 10.5% per blood unit (BU) of “CMV unsafe” blood, being directly proportional to the number of units transfused. The risk of TT-CMV can reach up to 60% in multitransfused individuals (i.e., those receiving at least 10 BU) [Harmening, 2006; Medeiros et al., 2007; Wu et al., 2010].
The reactivation of latent virus has been associated with immunosuppression, however, it can also occur in immunocompetent individuals [Naucler, 2006; Cook, 2007; Kalil and Florescu, 2009]. CMV infection plays a role in disease progression in several chronic hematologic disorders such as hemophilias, sickle-cell and aplastic anemia, hemoglobinopathies, lymphomas, and myelomas [Haddad et al., 1984; Torok-Storb et al., 2001; Manna et al., 2003; Ortin et al., 2005; Ocak et al., 2006; Ho, 2008; Crough and Khanna, 2009]. In these patients, CMV may be associated with severe infection affecting gastrointestinal tract (colitis), central nervous system (meningitis, encephalitis, and transverse myelitis) and lungs (pneumonia), with ability to cause fatal infection [Manna et al., 2003; Rafailidis et al., 2008; Abgueguen et al., 2010].
Few studies have assessed the CMV seroprevalence and the infection profile in hematologic disorder patients (HDPs), who are often exposed to the virus via blood transfusions. Such information could be very useful for defining some management strategies of these patients. This study evaluated the serological profile of anti-CMV antibodies and two acute-phase reaction proteins in HDPs from the Haematology and Haemotherapy Foundation of Bahia State, Brazil (HEMOBA).
MATERIALS AND METHODS
This work was a cross-sectional study. Retrospective data were also used to characterize the patients. The research protocol was approved by the Ethics Committee of the Bahia State Health Department (No. 256/2008).
All participants were volunteers who, after being briefed about the aims of study, signed an informed consent form. The research was carried out between August 2008 and March 2010.
Sample
A total of 470 HDPs from the ambulatory department of the HEMOBA, a referral-based hemotherapy service for the entire state, were enrolled in the study.
Eight milliliters of blood were collected from each patient and deposited into anticoagulant-free tubes with gel separators (BD Vacutainer® SST™, Franklin Lakes, NJ). The retrospective data were obtained through analysis of clinical records.
Laboratorial Analysis
Anti-CMV IgM and IgG antibodies were assayed based on immuno-chemiluminescence using a LIAISON automated system (DiaSorin SA, Sallugia, Italy) according to the manufacturer´s instructions. Extreme IgG anti-CMV values [30-fold IgG cut-off (≥15 IU/ml) or negative] and/or positive anti-CMV IgM values were used when confirmed in duplicate.
In all serum samples positive for anti-CMV IgM, anti-CMV IgG avidity immunoassay were performed in duplicate using a LIAISON automated system according to the manufacturer´s instructions.
Serological levels of high sensitivity C-reactive protein (CRPH) and alpha-1-acid glycoprotein (AAG) were measured using immunonephelometry techniques with an IMMAGE® Immunochemistry System (Beckman Coulter, Inc. Brea, CA) according to the manufacturer´s instructions.
Statistical Analysis
Statistical analysis was performed with SPSS® 9.0 for Windows and GraphPad InStat/Prism 3.03. Categorical variables (gender, age groups, hematologic disorders, units transfused, prior surgery, home address, and positive serology) were analyzed using the chi-squared statistical test with Yates continuity correction, accepting differences as statistically significant when P < 0.05 in two-sided analysis.
The normality of continuous variables (CRPH and AAG levels) was analyzed by the Kolmogorov–Smirnov test. Neither of the parameters was normally distributed. Logarithmic transformation was sufficient to make AAG data normally distributed, and an unpaired Student's t-test was performed to compare AGG means between CMV negative and positive groups. CRPH data were analyzed using the unpaired nonparametric Mann–Whitney test.
RESULTS
The sample was composed of 238 (50.6%) males and 232 (49.4%) females, aged between 0 and 95-year old (mean = 32, median = 29) and uniformly distributed among the defined age groups. The overall seroprevalence of CMV was 89.4% and was directly proportional to age and to the amount of BU transfused (Table I).
| Variables | CMV+ | CMV− | P-value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Gender | |||||
| Male | 207 | 87 | 31 | 13 | 0.12 |
| Female | 213 | 91.8 | 19 | 8.2 | |
| Age groups (years) | |||||
| 00–08 | 47 | 81 | 11 | 19 | 0.04 |
| 08–18 | 59 | 74.7 | 20 | 25.3 | <0.01 |
| 18–28 | 87 | 90.6 | 09 | 9.4 | 0.79 |
| 28–38 | 67 | 94.4 | 04 | 5.6 | 0.20 |
| 38–48 | 54 | 91.5 | 05 | 8.5 | 0.73 |
| 48–58 | 44 | 97.8 | 01 | 2.2 | 0.09 |
| >58 | 62 | 100 | 00 | 00 | <0.01* |
| Hematologic disorder | |||||
| Sickle-cell anemia | 148 | 90.2 | 16 | 9.8 | 0.77 |
| Other anemias | 56 | 78.9 | 15 | 21.1 | <0.01 |
| Cancer | 39 | 97.5 | 01 | 2.5 | 0.14 |
| Hemophilia | 47 | 85.5 | 08 | 14.5 | 0.44 |
| Hemoglobinopathy | 57 | 93.4 | 04 | 6.6 | 0.38 |
| Other | 73 | 92.4 | 06 | 7.6 | 0.45 |
| Units transfused | |||||
| 0 U | 50 | 56.2 | 39 | 43.8 | <0.01 |
| 00–05 U | 262 | 96 | 11 | 04 | <0.01 |
| 05–10 U | 73 | 100 | 00 | 00 | <0.01* |
| 10–15 U | 18 | 100 | 00 | 00 | 0.27* |
| 15–20 U | 07 | 100 | 00 | 00 | 0.76* |
| >20 U | 10 | 100 | 00 | 00 | 0.56* |
| Prior surgery | |||||
| Yes | 104 | 91.2 | 10 | 8.8 | 0.56 |
| Not | 314 | 88.7 | 40 | 11.3 | |
| Home address | |||||
| Bahia's capital | 151 | 95 | 08 | 05 | <0.01 |
| Bahia's interior | 269 | 86.5 | 42 | 13.5 | |
| Positive serology | |||||
| Anti-HIV-1/2 | 02 | 100 | 00 | 00 | 0.62* |
| Anti-T. pallidum | 06 | 100 | 00 | 00 | 0.85* |
| Anti-T. cruzi | 09 | 100 | 00 | 00 | 0.62* |
| AgHBs | 00 | 00 | 00 | 00 | ** |
| Anti-HCV | 15 | 88.2 | 02 | 11.8 | 0.88 |
| Anti-HTLV-I/Il | 09 | 81.8 | 02 | 18.2 | 0.74 |
- U, blood units.
- * To make calculation possible, 0.5 was added to each value.
- ** Chi-squared analysis was impossible.
Among the observed hematologic disorders (sickle-cell anemia, other anemias, cancers, hemophilias, hemoglobinopathies, and others), CMV seroprevalence was lower (78.9%) in the group designated “other anemias” (P < 0.01; Table I). Only four (0.9%) HDPs were serum reactive for anti-CMV IgM, three had moderate avidity anti-CMV IgG, and one was seronegative for anti-CMV IgG (data not shown). HDPs whose home address was in Salvador City, the capital of Bahia State, exhibited a higher seroprevalence of the virus (P < 0.01; Table I).
Patients were stratified, in accordance with Matos et al. [2010], into four groups according to anti-CMV IgG serological titers. It was observed that most CMV seropositive HDPs had anti-CMV IgG in low titers (0.45–5.00 IU/ml; Fig. 1). There was a statistically significant difference between gender in the low titers (51.3% vs. 35.3%, P < 0.001) and high titers (16.8% vs. 32.3%, P < 0.001) groups. There were differences between females versus total in low titers (35.3% vs. 43.4%, P = 0.04) and high titers (32.3% vs. 24.5%, P = 0.03) and males versus total in high titers (16.8% vs. 24.5%, P = 0.03; Fig. 1).
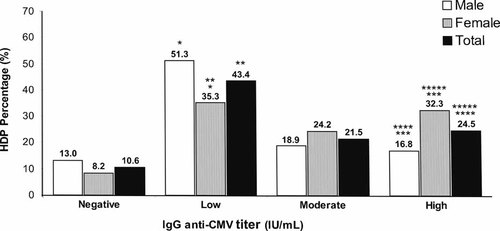
Percentage (%) of anti-CMV IgG groups according to gender. (Negative = <0.45 IU/ml; low = 0.45–5.00 IU/ml; moderate = 5.00–15.00 IU/ml; high = >15.00 IU/ml) *P < 0.001; **P = 0.04; ***P < 0.001; ****P = 0.03; *****P = 0.03.
There was no statistical difference in the AAG means between groups (P = 0.15; Fig. 2). The AAG mean in CMV negatives was 100.25 mg/dl (log10AAG: mean = 1.96; standard deviation = 0.19; median = 1.96; range = 0.83). In CMV positives, the AAG mean was 116.28 mg/dl (log10AAG: mean = 2.01; standard deviation = 0.22; median = 1.96; range = 1.00; Fig. 2).
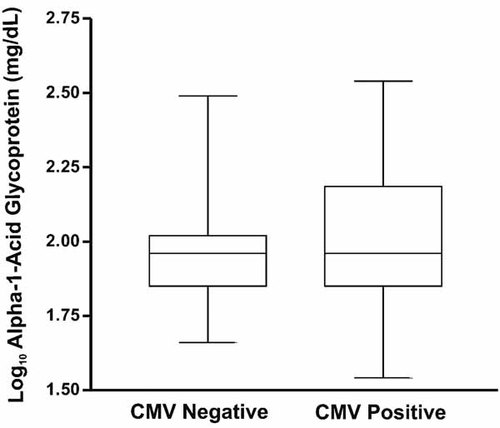
AAG levels according to CMV serostatus.
A statistical difference was observed in mean serum CRPH concentrations between groups (P < 0.001; Fig. 3). The mean CRPH concentration in CMV negatives was 0.57 mg/dl (standard deviation = 0.85; median = 0.30; range = 3.87), while in CMV positives the mean serum CRPH concentration was 2.70 mg/dl (standard deviation = 4.83; median = 0.57; range = 25.68; Fig. 3).
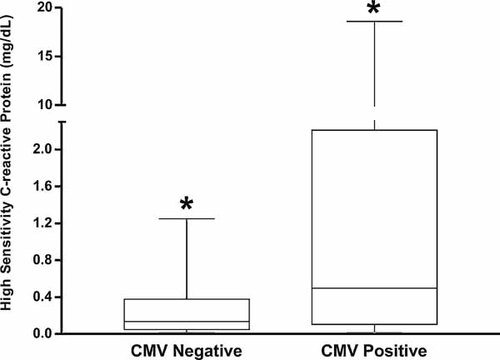
CRPH levels according to CMV serostatus. *P < 0.001.
DISCUSSION
The overall CMV seroprevalence in HDPs was found to be 89.4%. It was directly proportional to age and the number of BU transfused (Table I), which is in agreement with the findings of Staras et al. [2006], Medeiros et al. [2007], and Crough and Khanna [2009]. Of the 4 (0.9%) HDPs that were anti-CMV IgM positives, only 1 case could be characterized as recent acute infection due to seronegativity for anti-CMV IgG [Dangel et al., 2006; Cavlek et al., 2008; Kanengisser-Pines et al., 2009].
The high seroprevalence of CMV that was discovered suggests that there is a wide circulation of the virus in the Bahia State; however, this prevalence is consistent with other Brazilian, as well as worldwide, studies. For example, serological surveys among blood donors showed the following regional CMV seroprevalences: 87.9% in Brazil [Matos et al., 2010], 93.2% in Ghana [Adjei et al., 2006], 95% in India [Kothari et al., 2002], 97.2% in Turkey [Mutlu et al., 2008], 92% in Nigeria [Alao et al., 2008], 97.14% in Tunisia [Gargouri et al., 2000], 69.8% in Spain [Rojo et al., 1992], and between 35.5% and 45.8% in the USA [Hecker et al., 2004; Boeke et al., 2008].
Of all the hematologic disorders observed, the seroprevalence of CMV was lowest in the “other anemias” group (P < 0.01; Table I). In the other disorders studied (sickle-cell disease, hemoglobinopathies, cancers, hemophilias, and others) the seroprevalence was consistently above 85%.
According to Sabin et al. [2000], CMV seropositivity was associated to AIDS progression in HIV-infected patients with hemophilia. A group of Turkish researchers showed that patients undergoing hemodialysis had higher seropositivity for CMV (99.6%) than a control group (82.9%); this led the researchers to suggest improving the monitoring of dialysis patients by performing serological tests to identify those who are susceptible to CMV infection [Ocak et al., 2006].
Seropositivity for CMV should be seen as an important data for patients with hematologic disorders, since the reactivation of the virus may be associated with severe infection and death [Haddad et al., 1984; Ortin et al., 2005; Kalil and Florescu, 2009]. Some published studies was found that the virus may play a role in disease progression or even as an important co-infecting agent in several diseases such as cancers [Baldauf et al., 1996; Wrensch et al., 2001; Michaelis et al., 2009], renal diseases and hemophilias [Ocak et al., 2006], sickle-cell anemia, and other hemoglobinopathies [Haddad et al., 1984], aplastic anemia [Torok-Storb et al., 2001], AIDS [Ho, 2008; Crough and Khanna, 2009], and lymphomas and myelomas [Manna et al., 2003; Ortin et al., 2005].
Manna et al. [2003] reported three cases of CMV severe infection in patients affected by hematological malignancies (non-Hodgkin lymphoma or myeloma) who developed pneumonia and died. The authors point out that CMV infection is a severe condition that needs to be considered in patients with hematologic malignancy presenting with fever or pneumonia.
Rafailidis et al. [2008] retrieved 89 articles reporting on severe CMV infection in 290 immunocompetent adults. The gastrointestinal tract (colitis) and the central nervous system (meningitis, encephalitis, transverse myelitis) were the most frequent sites of severe CMV infection. Among immunocompetent patients, 25 were identified as having hematologic disorders caused by CMV infection. These disorders included: symptomatic thrombocytopenia, hemolytic anemia, disseminated intravascular coagulation, myelodysplastic changes, pancytopenia, and splenic rupture. This group of patients presented with a diversity of symptoms, including fatigue, fever, abdominal or chest pain, headache, pain in the extremities, numbness of the hands, darkening of urine due to the presence of hemoglobin, epistaxis, easy bruising, purpura, increased incidence of infections, jaundice, and systolic ejection murmur.
Medeiros et al. [2007] evaluated the use of real-time PCR for the detection of CMV in blood donors at the Haemotherapy Center of Para State, Brazil. Viral DNA was detected in 57% of blood donors tested, which lead the authors suggest the use of this trial test when selecting blood to be transfused in immunocompromised patients and women in the early stages of pregnancy.
It was observed that HDPs who received transfusions of more than 1 BU had higher CMV seroprevalence than those who had not received any transfusions (96% vs. 56.2%, P < 0.001; Table I); however, it is important to note that the average age of the HDPs that had not received transfusions was lower than those that had, a factor that also contributed to the lower seroprevalence. Nevertheless, this difference in CMV seroprevalence between the groups reinforces the importance of using strategies such as leukoreduction and/or transfusion of CMV-negative blood in patients at high risk of developing severe CMV infections. There was no statistically significant difference (P > 0.05) in CMV seroprevalence between HDPs who received more than 1 BU.
The importance of blood banks maintaining an inventory of CMV-negative blood in order to be able to give safe transfusions to high-risk patients is much discussed in literature; however, obtaining CMV-negative blood from donor populations where CMV prevalence is high presents a significant challenge [Pamphilon et al., 1999; Qu and Tran, 2007; Adjei et al., 2008; Fontaine et al., 2010].
Additionally, Table I shows that HDPs with home addresses in the capital of Bahia State, Salvador City (HDI = 0.746), showed higher rates of CMV seroprevalence than observed in the other cities of the state (95% vs. 86.5%, P < 0.01). It is particularly worth emphasizing that CMV seroprevalence is inversely proportional to socioeconomic status [Junqueira et al., 2008; Crough and Khanna, 2009]. The prevalence of serological markers for other blood transmitted infectious diseases was in accordance with national estimates [Salles et al., 2003] and did not differ between CMV positive and negative groups (P > 0.05).
Some studies showed different levels of anti-CMV IgG antibodies in the seropositive population, with titers of anti-CMV IgG differing up to 50-fold between individuals [Gargouri et al., 2000; Kanengisser-Pines et al., 2009]. Bonon et al. [2006] observed that during viral reactivation, the individuals increase anti-CMV IgG titers; this led the researchers to conclude that, in the absence of antigen detection immunoassays, serology could be useful as a tool to monitor infections.
The present study further investigated this possibility by categorizing HDPs according to the serum titers (negative, low, moderate, and high) of anti-CMV IgG antibodies (Fig. 1). Among CMV seropositives, the most prevalent IgG group were low titers (0.45–5.00 IU/ml), which usually is observed in latent and controlled infection. The presence of moderate and high titer groups may be related to the infection instability, which is associated with hematological disorders, blood transfusions, immunosuppression, and even use of medications. When analyzed according to gender, there was difference in the percentage of anti-CMV IgG level groups in low titers (51.3% vs. 35.3%, P < 0.001) and high titers (16.8% × 32.3%, P < 0.001), showing a tendency for females to have higher anti-CMV IgG titers than men (Fig. 1), results similar to those observed in blood donors by Matos et al. [2010].
The immune response to CMV infection involves cells, antibodies, and inflammatory mediators such as the acute phase reaction proteins. The acute phase reaction proteins most commonly used as inflammation biomarkers are as follows: C-reactive protein (CRP), AAG, fibrinogen, C3, haptoglobin, and alpha-1-antitrypsin [Packard and Libby, 2008; Costalonga et al., 2009]. Blood levels of CRP are known to rise rapidly from normal baseline levels of <0.3 mg/dl to levels as high as 50 mg/dl as part of the body's nonspecific inflammatory response to infection or injury [Dati et al., 1996]. CRP is currently the most thoroughly validated inflammatory biomarker, however, others are being increasingly studied [Packard and Libby, 2008; Costalonga et al., 2009].
Some works have highlighted the association between serum CRP levels and CMV infections. Nubling et al. [2003] observed slightly elevated levels of CRP during an active CMV infection in immunocompetent patients. Muhlestein et al. [2000] observed high mortality in CMV seropositive patients with coronary artery disease and elevated serological levels of CRP. Costalonga et al. [2009] observed an association between the severity of clinical manifestations of active CMV infections and elevated serum CRP levels.
Therefore, this study investigated the correlation between serum levels of both AAG and CRPH to CMV serostatus (Figs. 2 and 3). There was no difference between serum levels of AAG between CMV positive and negative HDPs (P = 0.15; Fig. 2). However, analysis of serum CRPH revealed that CMV positive HDPs had higher levels than CMV negative HDPs (0.57 ± 0.85 mg/dl vs. 2.70 ± 4.83 mg/dl; P < 0.001; Fig. 3). These data should be interpreted with caution, since there was a high degree of variability in CRPH serum levels between CMV positive HDPs, which may indicate that the hematologic disorders are the true cause of high values.
In conclusion, the results of this study are in agreement with others that have demonstrated the endemicity of CMV infection in Bahia State, Brazil. This highlights the importance of using strategies to provide “CMV safe” blood components to HDPs at high risk of developing severe infections, since the seroprevalence in these patients is high. CRPH can be used as a biomarker associated with CMV seropositivity; however, more efforts are needed to better characterize the clinical profile of active CMV infection in this group of patients.
Acknowledgements
We are grateful to the study participants and to the staff at the Haematology and Haemotherapy Foundation of the Bahia State, Brazil (HEMOBA), especially Dr. Virginia Figueiredo, Dr. Anelisa Costa, and Dr. Cláudio Brandão.




