Systematic review of MRI safety literature in relation to radiofrequency thermal injury prevention
Abstract
Introduction
Magnetic resonance imaging (MRI) is a rapidly evolving modality, generally considered safe due to lack of ionising radiation. While MRI technology and techniques are improving, many of the safety concerns remain the same as when first established. Patient thermal injuries are the most frequently reported adverse event, accounting for 59% of MRI incidents to the Food and Drug Administration (FDA). Surveys indicate many incidents remain unreported. Patient thermal injuries are preventable and various methods for their mitigation have been published. However, recommendations can be variable, fragmented and confusing.
The aim of this systematic review was to synthesise the evidence on MRI safety and associated skin injuries and offer comprehensive recommendations for radiographers to prevent skin thermal injuries.
Methods
Four journal databases were searched for sources published January 2010–May 2023, presenting information on MRI safety and thermal injuries.
Results
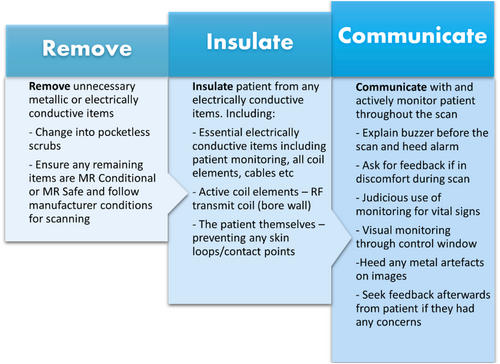
- Remove any electrically conductive items
- Insulate the patient to prevent any conductive loops or contact with objects
- Communicate regularly
Conclusion
By implementing the above recommendations, it is estimated that 97% of skin burns could be prevented. With thermal injuries continuing to impact MRI safety, strategies to prevent skin burns and heating are essential. Assessing individual risks, rather than blanket policies, will help prevent skin thermal injuries occurring, improving patient care.
Introduction and rationale
Magnetic resonance imaging (MRI) is considered a relatively safe imaging modality, which produces excellent soft tissue contrast, using no ionising radiation unlike X-ray or computed tomography (CT).1 It is not without safety issues, however, with patient burns being the most frequently reported adverse event (59% of reports to the Food and Drug Administration (FDA)2 and 47% to the Medicines and Healthcare products Regulatory Agency (MHRA)3). These burns can be caused by; radiofrequency (RF) deposition, eddy currents, contact with an object (unsafe or incorrectly used electrically conductive item), contact with the individuals own skin, or even the bore wall.4
Published case reports of MRI burns exist from as far back as 1989.5 These burns are unpredictable and while some are instantaneous after MRI exposure, other significant burns may manifest even 24 h after MRI scanning.6, 7 Severity ranges from 1st to 4th degree burns, such as small blisters8 up to severe burns requiring amputations.9 Table 1 below summarises numbers of reported thermal injuries globally over different time periods and some of the commonly assumed causes. However, Kihlberg et al.10 found MRI incidents are severely underreported by 1/3 and Rogg11 suggests this is due to voluntary reporting, indicating potentially many more unreported incidents. Gilk and Kanal12 analysed adverse events reported to the FDA in 2009 and 2010 and found that 97% of burns may have been prevented following ACR guidance from 2007.13 The ACR document is one of many published by various MRI safety advisory bodies that radiographers use to guide practice,14-16 however, the advice and recommendations are not required to be followed as MRI safety practise is not mandated.12 There have been reviews of patient burns which have been reported to, and recorded by, organisations' mandatory safety reporting systems2, 7 and there are many studies into the theoretical mechanisms of such burns.17, 18 Tang and Yamamoto4 have investigated the factors associated with MRI related burns. However, to date, no comprehensive review of international safety recommendations to prevent MRI induced skin burns exists.
| Country year (reference) | United States 1997–20097 | United States 2008–20172 | United Kingdom 1993–201419 | United Kingdom 2012–20213 | Japan < −20104 | Australia 2010–2022 | Denmark 2015–201720 |
|---|---|---|---|---|---|---|---|
| Contact with an object | 58 | 257 | 53 | 11 | 79 | 7 | - |
| Skin-to-skin contact | 46 | 147 | 39 | 31 | 49 | 7 | - |
| Bore contact | 105 | 97 | - | - | 12 | 1 | - |
| Unclear | 150 | 348 | 39 | 55 | 9 | 14 | 5 |
| Total events | 419 | 849 | 131 | 97 | 149 | 29 | 5 |
| Total MRI exams this period (reference) | 278 million21 | 335 million21 | 26 million from 1995 to 201422 | 28 million in NHS England22 | 14 million in 2014 alone21 | 10 million21 | 1.4 million21 |
This article focuses on the risk of skin burns and heating associated with patient positioning causing contact with either external electrically conductive items, the patient's own skin or bore wall. It is beyond the scope of this article to address implant heating, interventional or special MRI applications (Radiation Therapy planning/or coupled with Positron Emission Tomography), field strengths over 3 T, vertical fields or core temperature changes.
Aim
The aim of this systematic review is to synthesise published information on MRI burns and safety-related guidance to develop comprehensive safety recommendations for best MRI practice to prevent burns. This article aims to guide radiographers, using published best practice documentation, to make, patient tailored, informed decisions.
Methodology
A systematic review was performed across multiple databases and grey literature sources to obtain all literature associated with MRI safety and burns. This included the physical principles and explanations for burns caused by MRI, and general guidance, policy statements, original research, protocols, recommendations, reviews and case reports.
Protocol
This systematic review was conducted with guidance from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.24 The review was registered with PROSPERO.23 The database search was initially run on 9th March 2022 and repeated on 17th May 2023. Two reviewers performed title and abstract screening using Covidence25 with 100% agreement (0 conflicts). The second reviewer suggested expanding the inclusion criteria to include case reports as these are often used by the radiographer community as a reference source. After first reviewer completed full-text review, the second reviewer repeated this on a selected sample of 32% of the data with 0 conflicts. Data extraction was completed by the first reviewer only.
Sources of Information
The selected databases were PubMed, Scopus, CINHAL and Science Direct. Guidelines and manuals were further sourced in grey literature, related textbooks and online sources. These databases were selected for their strong medical and scientific content.
Eligibility criteria
Eligibility criteria applied to the search were limited to English language and publication date from 01 January 2010 until 17th May 2023. This start date was selected as a ‘neat’ date and then final date was just prior to final data analysis. Full inclusion and exclusion criteria are shown below in Table 2.
| Inclusion | Exclusion | |
|---|---|---|
| Study type |
|
|
| Type of MRI |
|
|
| Primary Theme |
|
|
| Population demographics |
|
|
| Types of outcomes |
|
|
| Language |
|
|
| Dates |
|
Search strategy
The search strategy concepts were ‘MRI’ and ‘safety’ or ‘burns’. The key concepts were expanded to include search terms shown in Table 3 and combined with Boolean operators with relevant truncation and adjustments for each databases's thesaurus (MeSH terms vs. subject headings). The words ‘best practice’, ‘guideline’, policy, ‘protocol’ and ‘white paper’ were not used as the inclusion of these could have excluded physics and reference material not containing these phrases. Full electronic search strategy for PubMed is available on request. The search was open to include case studies, qualitative papers and the preferred results would include white papers. Further refinement was applied during screening using the inclusion and exclusion criteria. De-duplication was performed in Covidence, a web-based systematic review management software.25
| Concept 1 all OR | AND | Concept 2 all OR | |
| Concept | MRI | Burns | |
| Keywords used [Title/Abstract] |
MR imaging Magnetic resonance imaging Magnetic resonance MRI |
Burn Safety Adverse event Adverse incident Thermal effect* Thermal injur* Rf burn EMF burn |
|
| MESH terms [MeSH] | Magnetic Resonance Imaging |
Burns Patient safety |
|
| Limiters |
(“2010/01/01”[Date – Publication]: “2023/05/17”[Date - Publication]) AND (English[Language]) |
||
Study selection and synthesis
Screening was performed in Covidence25 at title and abstract level, with criteria shown below in Table 2 There were no population-specific demographics specified, as burns have been reported from young children9 to adults26 and the same burn prevention strategies would also apply to animals in a veterinary or research situation.
Articles that were deemed relevant were then searched as full text against the eligibility criteria. The selected studies had to either be focused on MRI burn prevention or MRI safety.
Grey literature provided the remainder of the results. This included hand searching of reference lists, as well as dedicated searching of relevant industry publications, professional bodies' and MRI learned societies' guidance and recommendations. The International Society for Magnetic Resonance in Medicine (ISMRM)27 list of international MRI safety documentation was also explored. Several primary textbooks were also reviewed and relevant chapters on safety were included. A final review by the authors was performed to ensure no important documents were omitted.
Data extraction and synthesis methods
- Change into gown or scrubs
- Remove unnecessary metallic or electrically conductive items
- Only trained staff should use and scan only MR acceptable items, following manufacturer instructions for MR conditional devices
- Follow MR Safe/Conditional criteria for implants/devices
- Check integrity of all equipment, remove any damaged/unplugged/unused equipment, maintain equipment
- Positioning of necessary electrically conductive materials
- Padding to avoid contact; skin-to-skin, bore wall, electrically conductive materials
- Awareness of resonant length conductors – specific to each field strength
- Use lowest possible SAR
- Monitor the patient during the MR procedure and respond to appropriate alerts
- Recognise metal image artefacts early
- Monitor and alert patients to concerns related to tattoos
After extraction was completed in Covidence, data was exported to a spreadsheet for data synthesis. Themes were identified using thematic analysis and similar recommendations were combined to create a final nine themes. Responses for each of the nine themes were copied to individual sheets as distinct data sets and filtered for positive responses. These were then analysed, and commonalities were coded using thematic analysis.31
Risk of bias assessment
The risk of bias assessment was performed using the Joanna Briggs Institute (JBI) Checklist for Text and Opinion and applied within the Covidence software.32 This checklist was suitable for the search results which contained a variety of papers including reviews, white papers and text and opinion pieces.33 Each of the six elements of the checklist were entered into Covidence and during extraction, ‘yes’, ‘no’, ‘unsure’ or ‘not applicable’ were selected and a final decision of include or exclude were selected.
Results
Study selection
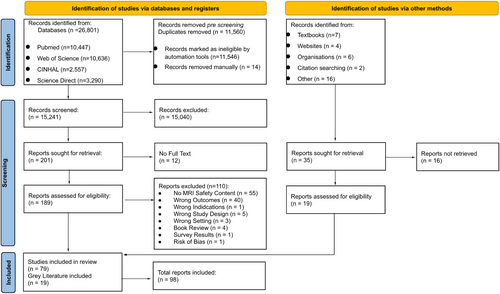
Total search results were 26,801 and 15,241 after duplicates removed. A detailed PRISMA flow diagram can be seen in Figure 1. Title and abstract screening reduced this to 201 for full-text review. After full-text review, 79 peer-reviewed papers were included, and an additional 19 grey literature sources resulted in 98 total sources.
Study characteristics
The key study characteristics are available in tabular format in Table S1. Of the final 98 sources included, 23 were considered guidance documents, manuals or recommendations, 23 were reviews, 11 were textbook chapters, 3 were commentaries, 5 were text and opinion while there were 7 experimental and 3 quantitative studies, 22 case reports and 1 other. Refer to Table 4 for the study characteristics. The publication year range was from 2010 to 2023 and included studies were from USA (n = 50), other countries (Belgium, Canada, Denmark, Europe, Finland, France, Germany, India, Jordan, Korea, Macedonia, New Zealand, South Africa, The Netherlands and Turkey) (n = 28), United Kingdom (n = 10), Australia (n = 5), and Japan (n = 5). Most papers were written in relation to human imaging (n = 89) while others were written about animal imaging (n = 3), phantoms (n = 3) and results from virtual human simulations (n = 3). Of the papers written about human imaging, there was one dedicated to neonatal imaging.
| Literature type | Number of sources (References) |
|---|---|
| Guidance documents, manuals or recommendations | 23(6, ) |
| Reviews | 23(1, 2, 4, 11, 30, 37, 43, 66, 73, 94, 111, 112, 117, 120, 121, 123, 124, 126, 128, 133, 135, 154, 155) |
| Case Reports | 22(8, 26, 35, 36, 52, 59, 87, 90, 93, 95, 96, 136, 137, 140-143, 145-148, 152) |
| Text book chapters | 11(29, 44, 47, 69, 106-109, 117, 129, 130) |
| Experimental studies | 7(18, 82, 114, 138, 149, 151, 153) |
| Quantitative studies | 3(144, 150, 159) |
| Text and opinion | 5(67, 115, 134, 156, 157) |
| Commentary | 3(76, 120, 122) |
| Other | 1(101) |
Risk of bias results
The risk of bias results are available upon request. One article was excluded during critical appraisal due to insufficient reference to extant literature. All other sources met criteria sufficiently to be included.
Data synthesis results
During data synthesis, the sourced papers fitted in the following nine primary themes as can be seen in Table 5 above.
| Primary Theme | Results |
|---|---|
| Change patient into gown |
44% recommended gowns 19% were concerned about metallic fibres in the fabric 11% were concerned with metallic components (zips, clasps etc) 4% were concerned about pockets and potentially hidden items |
| Remove unnecessary metallic or electrically conductive items | 64% of papers discussed the process of acknowledging and removing excess items |
| Check integrity of all equipment |
21% mentioning (either or both) removing damaged/unused equipment and/or checking or maintaining equipment 18% mentioned damaged coils/insulation 4% instructed to remove unplugged equipment 4% advised to remove equipment if it was not working 16% recommended regular inspection/maintenance |
| Positioning of necessary electrically conductive equipment |
35% had recommendations for the positioning of necessary electrically conductive items 12% mentioned keeping electrically conductive materials down the centre of the bore in the z axis 22% recommended avoiding loops 28% mentioned preventing cables touching the patient 6% specifically mentioned external fixation or prosthesis |
| Awareness of resonant length | 18% mentioning resonant length or frequency |
| Patient padding |
55% discussed padding to prevent ‘something’ contacting the patient 46% of papers recommended avoiding skin-to-skin contact or closed loops 32% recommended padding between the patient and the bore/transmit RF coil 13% recommended padding between the patient and electrically conductive materials including coils, cables, monitoring etc |
| Monitor patient | 45% included instructions to monitor patient |
| Awareness of artefacts | 15% warned about unexpected artefacts |
| Awareness of tattoos | 38% mentioned tattoos |
Discussion
There was limited consensus between all 98 sources found in the systematic review. Using scientific rationale guided by the results of the systematic review, the following sections will endeavour to provide guidance for radiographers to prevent RF-induced patient burns.
Change patients into a gown
There were many papers concerned about the risk of clothing containing metallic fibres to cause patient burns.35-37 The increasing use of silver or copper for their antimicrobial properties in athletic wear, underwear and socks increases the likelihood of MRI patients to be wearing such materials. While silver and copper are non-ferromagnetic, these materials are excellent electrical conductors and therefore the risk of induced current and subsequent heating does exist.38 It should be noted that there are three distinct versions of fabric technology. One is where the silver fibres are part of the weave and components of the manufacturing process, while the other options are a ‘treatment’ to where either the polymer fibres or, the completed fabric is sprayed with microscopically small silver or copper ions.39 There is yet to be any research to demonstrate the difference between these in terms of burns risk. As there is no requirement for manufactures to disclose impurities and no way to detect these fibres or fabric treatments, changing into facility approved gowns or scrubs is an effective way to reduce the risk of a fabric-induced burn.37 There is also a risk of heat-trapping with excess or inappropriate clothing, hindering a patient's normal thermoregulatory cooling system.26, 37
The recommendation to change into a gown or pocketless scrubs also avoids other metals potentially fixed to clothing, including fasteners. Pocketless scrubs will also remove the risk of unknown items carried in pockets.43
It is generally considered that there is minimal risk of burns from small, non-ferrous metallic fasteners such as buttons less than 2 cm but they can cause artefact on the images.15, 40 The FDA testing manual does not require testing of passive implants less than 2 cm and more than 3 cm from another passive implant.40 However, this exclusion is not valid if part of the implant is outside the patient due to the heat conduction properties of the body.40 Metal zips, if within the region of RF, may match the appropriate wavelength of the transmitted RF to result in resonance and induce heating in the conductor.42 There are also versions of wearable technology where the heart rate and respiratory sensors are built into the clothing.41 These would pose risks of burns from the antennae effects (resonance), device malfunction and image artefact.
The risk of burns due to clothing is only present when all or part of the item is exposed to the RF field and while many sources recommended changing into a gown, a facility may choose to only have patients remove clothing in or near the area exposed to RF during that scan.14, 43 However, this may lead to patient confusion at subsequent examinations and a consistent policy reduces the risk of non-compliance.
Remove all unnecessary metallic or electrically conductive items
Contact with an object accounted for 257 thermal injuries reported to the FDA database between 2008 and 2017.2 Many non-essential items should be removed for reasons beyond RF burns risks (such as induced currents due to exposure to gradient magnetic fields) and will not be further addressed.
Ferromagnetic detectors (FMDS) are increasingly seen as a tool to be used as an adjunct or ‘final check’ to ensure all ferrous materials have been removed prior to entering the magnet room.14 The ACR manual and MHRA safety guidelines recommend the use of FMDS as opposed to conventional metal detectors.14, 15 These reduce the risk of projectiles and can detect some implants. However, there are many materials that are non-ferrous yet still electrically conductive, including silver, copper and carbon fibre.44 FMDS may provide a sense of security regarding the prevention of ferrous items entering the magnet room, but if non-ferrous items remain undetected, burns may still occur from this type of conductor.37, 45 FMDs are an adjunct to and “not a replacement for a thorough screening practice” (14p12).
There is no consensus regarding wearing of jewellery. A practical approach would be to remove all jewellery where possible but if the patient is unable to easily remove it, permit non-ferromagnetic metals to be worn when not exposed to RF energies.46 If exposed to RF, items should be assessed case by case.47
There are wearable technologies designed to appear as rings48-50 that would be considered MR Unsafe due to electronics that could be damaged. Electrically conductive loops can be a cause of heating.51 As the heating effects of such small calibre loops, when exposed to electromagnetic fields, has been untested such devices should be removed. This would also eliminate any potential for image artefacts caused by such devices.
Hair extensions can contain metals and should be treated with caution as they may provide conditions for RF heating.52, 53
Drug and medication patches had highly variable recommendations. Applying a purely scientific approach, any patch not exposed to the RF poses no burns risk by either conduction, antennae or near field effects.6, 54 A recent review55 found that of 56 FDA-approved dermal drug patches, 13 had some metallic component while 6 had insufficient information to determine if metallic components were present. A site-specific protocol should be used. Guidance recommends medication patches should only be removed after consultation with the prescribing physician.14, 16, 56 On a practical level, informed patient consent and a cold compress may prevent excessive heating and increased drug delivery; however, caution remains around cooling strategies such as ice packs which may lead to a reduced drug delivery rate.14
Different studies have shown antimicrobial silver dressings to be safe to scan up to 3 T thus do not need to be removed, even if in the area of interest.57, 58 However, the risks of thermal injury when large electrically conductive antimicrobial dressings are in place has not been tested and may warrant further research. There is one reported case of a burning sensation in the presence of zinc oxide ointment treatment where the scan was stopped before any burn could occur.59
A recent surge in popularity of continuous glucose monitoring (CGM) and Flash Glucose Monitoring (Flash GM) for diabetes and even health and fitness purposes60 have added another risk. Most of these devices are MR Unsafe and must be removed prior to scanning.61 These devices typically have a 10-to-14-day use period and cannot be reused once removed.62, 63
There is also a lack of research regarding the heating risks of these devices. Adaptations to booking processes may be necessary to optimise booking the patient's scan to the end of their device's cycle to reduce unnecessary disposal.
Infectious diseases such as Covid-19 has highlighted the more frequent use of facemasks. If masks contain metal, they pose RF heating risks and some have been felt to be MR unsafe64 and should be replaced with masks with no metal.65
Most sources recommended removing unnecessary electrically conductive items wherever possible; however, there are some items that can be assessed on a case-by-case basis following department guidelines.
Check integrity of all equipment
This recommendation is designed to determine if equipment associated with the patient is properly maintained and to identify any damages or faults to ensure unsafe equipment is removed before scanning. Cracks and splits in insulation of cables can expose the patient to the internal wires and potentially create a conductive loop where burns could occur or even cause tissue or clothing to ignite.66 Appropriate maintenance of certain equipment is conducted by manufacturers and the ACR requires annual RF coil quality control for accreditation purposes.67-69 Damaged, or malfunctioning equipment should be removed from the patient and taken out of service until inspected and repaired.29, 47, 70, 71
It is also important to remove any unplugged equipment from the bore during image acquisition to ensure active and passive detuning circuits, that block resonance induction, are functioning to prevent decoupling failure, coil damage and burns.67, 72, 73
Adhere to conditions of all necessary electrically conductive materials
It is important that necessary electrically conductive items are MR Safe or MR Conditional and relevant instructions and conditions be followed.74 MRI instruction for use manuals further reiterate that “it is the obligation of the MRI operator to be aware of these conditions and to ensure that these conditions are strictly adhered to.”74 It is particularly pertinent for patient monitoring devices such as ECG and pulse oximetry. MRI conditional devices will have adaptions, such as fibre optics, shielding, carbon electrodes and plastic coatings, that ensure the electrically conductive components have reduced risk of burns when used as per manufacturer's instructions.75 All ECG and other monitoring equipment associated with the patient should be considered MR Unsafe until checked. Only approved MR Conditional ECG electrodes and monitoring devices should be applied as per manufactures instructions by MRI trained staff.70, 71
There were several cautions against using blanket policies for clearing patients and scanning outside device labelling. Input from medical physicists, MRI Medical Director's amongst others, with written consent from the patient, are recommended when developing such policies. Consideration of manufacturer's recommendations and adequate staff training are also necessary to ensure all equipment and devices are used in a safe manner.16, 76
The recommendation to position necessary electrically conductive materials along the centre of the bore is to avoid the large RF intensity found at the edges of the bore.30
It is also important to avoid forming any loops between electrically conductive materials. These materials could consist of ECG leads, coil cables, the scanner bore as well as the patient. These must be separated and insulated from each other16, 69 to prevent resonant circuitry72 and capacitive coupling.6, 70
External fixation devices such as those for lower limbs and halo collars for the cervical spine may be MRI Conditional, however, it is important to recognise that these may be electrically conductive and contact with any other wires or cables should be avoided.70 There were 11 reports of thermal injuries caused by stereotaxic headframes between 2008 and 2017.2
Awareness of unavoidable, potentially electrically conductive materials would also include surgical staples and bio-hacking implants such as RadioFrequency Identification (RFID) and Near Field Communication (NFC) devices. Provided the staples are non-ferromagnetic and outside the RF exposure field, the patient can be scanned without incident.14 However, if they are within the area of RF irradiation, warn the patient of potential heating and apply a heat sink.14 Patient bio-hacking involving NFC and RFID chips typically poses no major contraindications to MRI.77 Several studies have shown RFID chips to be MRI Safe or Conditional with no loss of function and no concerning levels of heating but potentially large artefacts.78-81 It should also be noted that RFID chips can be used as hospital ID, linen, access port or in breast implants and similar precautions should be used in these locations.82 Testing of Vivokey Spark 2 NFC devices found they pose no risk in MRI.83 Any bio-hacking implant containing a magnet must undergo case-by-case assessment as these are generally unsafe.84
Be aware of resonant length
Pad the patient to avoid contact burns
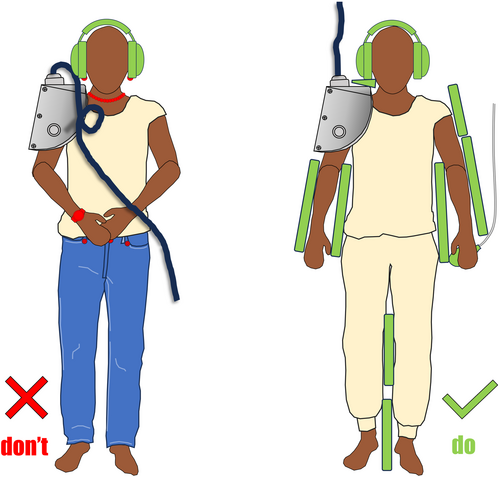
Skin-to-skin contact burns can happen anywhere on the patient, even beyond the RF field.86 Contact burns accounted for 147 reported injuries to the MAUDE database between 2008 and 2017.2 Fortunately, these are easily preventable.86 Common locations are between the legs8, 87, 88 or finger and thigh.89 These can be exacerbated by patient sweat and thus prevented by ensuring there is dry, water resistant, non-conductive material as guided by the manufacturer between any skin surfaces that may be in contact.88 These loop burns can occur outside the RF and Tang et al18 performed modelling for thumb to thigh contact and this was measuring above the local SAR limit of 10 W/kg up to 70 cm from the isocentre.
Padding to protect patients' skin from touching the bore is necessary to prevent burns from near field effects. Reports exist of patients burning elbows90 and the anterior abdominal wall.91 There are points along the wall of the bore where RF transmission is more concentrated, making burns more likely at these points if the patient is not properly insulated from the inner bore. For this effect, proximity to (rather than contact with) is critical so thicker insulation will work more effectively than a thin non-conductive pad. This heating may be exacerbated by large areas of contact not being able to cool through convection due to skin contacting the bore.92
Forensic analysis by lawyers into burn risks and informed consent has shown that proper padding is so effective when used correctly, there is no need to inform patients of burns risk, however, it would be prudent to reiterate the importance of the padding to the patient to prevent movement.93, 94
Monitor patients during scanning
Many superficial burns such as those associated with tattoos and skin-to-skin contact are felt instantaneously and the patient can alert the MRI radiographer to uncomfortable sensations promptly.94, 95 It is suggested that burns induced by antennae effect occur so quickly the patient may not react in time to prevent it, however, stopping the scan can reduce the severity.96 Conversely, burns associated with near field effects close to the bore often have a delayed reaction.6 There are case reports of burns where the patient felt discomfort during the procedure but did not alert the radiographers, assuming this was a normal sensation.8, 97 Other instances, however, describe how the patients' alerts were dismissed and after the scan, burns were evident.94 This emphasises the duty of care that radiographers have to communicate with their patients about potential risks and the importance of the call bell/buzzer if they have any discomfort.37
External monitoring can assist to monitor a patient's condition during the scan (especially when they are uncommunicative) but used incorrectly, can increase the risk of burns and thus should be used as per manufacturer's instructions. MRI manufacturer instructions recommend examining the patient between sequences to check for localised heating and potential burns.74
Be aware of artefacts as indicators of potential unknown foreign bodies
Radiographers should have an awareness of artefacts from the first localiser that appears on the screen. Artefacts could represent unknown foreign bodies, potentially electrically conductive materials or coil failures which could all potentially burn a patient.35 Radiographers should be aware of and investigate artefacts immediately to prevent any burns occurring.37
Be aware of tattoos and certain cosmetics exposed to the RF field
Tattoos have been linked with heating and RF burns.98 It is proposed that the mechanism through which this occurs is via the antennae effect.4 However, it has also been suggested that the ferromagnetic pigments of some tattoos are actually interacting with the static field.99 While 6 tattoo or permanent makeup incidents were reported to the FDA between 2008 and 2017,2 other studies found no burns.100, 101 A comprehensive literature review found only 17 published reports, all with full, fast recovery and no lasting sequelae.102 Tattoo reactions have been reported by patients instantaneously, so patients should be warned to squeeze the buzzer if any discomfort is felt and a cold compress may be applied if a tattoo will be exposed to RF.6, 95 If the patient had reduced communication capabilities, prophylactic application of a cold compress would be prudent.1, 14, 103, 104 However, this should not be a wet towel which can create a conductive loop and risk of burns via this mechanism.4
Summary
Analysis of the final 9 recommendations reviewed, found that the following condensed proposals to “Remove, Insulate and Communicate” (RIC), encompass the essential actions to prevent skin burns and should be implemented in MRI practice, as presented in Figure 2 and Figure 3 below. These recommendations are derived from the systematic review and the guidance from many MRI safety experts. Padding patients, insulating them from conductive materials, removing unneeded conductive items from the bore as well as positioning patients to prevent large calibre loops, has been promoted by Gilk and Kanal.12 They surmised that such actions could have prevented up to 97% of the burns that were recorded by the FDA over a 2-year period.12
Limitations
A systematic review of 98 different sources ranging from guidance manuals, textbooks, original research, white papers, articles, and virtual simulations provided a range of valuable independent recommendations. There were some overlaps with several distinct and reputable sources all containing similar content and the same author.29, 70, 71 These were all included as evidence of common places radiographers will search for recommendations. The intermittent occurrence of MRI burns makes controlled trials and other in vivo experiments difficult, however, there are experimental studies using various phantoms and virtual human models. These were still mostly excluded from results unless they reviewed, commented on or proposed guidance/recommendations for safe MRI practices. There were limitations to the scope of the reviewed recommendations. As mentioned previously, this paper does not discuss any implanted or active devices and only horizontal fields up to 3 T and is targeted at preventing skin burns only. While the underlying principles are the same, further assessments are warranted for any other scenarios. Some recommendations are based on consensus, while others were formed using virtual modelling or a scientific approach. Other limitations include the biases and inherent theoretical and epistemological commitments of the researchers performing thematic analysis.31 A further limitation was the lack of evidence for the mechanism of burns occurring due to metal in fabrics. Further research is required to investigate whether metal-infused fabrics or those containing metal fibres are more problematic. Controlled experimentation with thermal cameras, phantoms and monitoring equipment could be performed to demonstrate heating and how burns from these materials occur.
Conclusion
With thermal injuries accounting for the largest number of reported adverse events to regulatory bodies, strategies to prevent patient burns and heating are essential. Analysis of all recommendations reviewed and promoted are summarised with the acronym R.I.C., as described above. This could provide a simple method for training MRI staff on what actions are necessary to prevent patient skin burns caused by inappropriate practice.
Acknowledgements
The authors would like to thank Dr Emanual Kanal MD, FACR, FISMRM, MRMD, MRSE, AANG for reviewing this article. And Dr Frank G. Shellock, Ph.D., FACR, FISMRM, FACC, Director of MRI Safety and Adjunct Clinical Professor of Radiology and Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA for his encouragement of this project.
Funding information
There was no funding for this study.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




