Intra-operative duplex ultrasound scanning in renal transplantation: protocol and service requirements
Abstract
Intra-operative duplex ultrasound in renal transplantation was first described in 1998 and whilst reported in problematic cases, there are few reports of its routine use and no current published protocols. Since 2013, we have used intra-operative ultrasound in all renal transplants. The formal protocol used since August 2020 is presented as a reference document for other transplant centres. A Canon Aplio 800 ultrasound system with an i22LH8 hockey-stick transducer is used to image the renal cortex and major vessels, and an i8CX1 matrix transducer to image the graft during and after fascial closure. These transducers are fully sterilised with Sterrad and no sheathing of transducers is required. The transplant surgeon scans within the sterile field with the sonographer guiding imaging and adjusting machine settings. Ultrasound findings are discussed between team members including any requirement for interventions. Ultrasound is performed at three stages of the operation: Stage 1: after clamp release identifying issues of graft vascularity including otherwise unrecognised major vessel and anastomotic abnormalities. Stage 2: following ureteric implantation identifying compromised perfusion due to graft rotation or vessel kinking. Stage 3: after fascial closure identifying compromised perfusion due to external compression. Post-operative scanning, including assessment of the collecting system and bladder, is performed routinely on days 1, 3, 7 and 30. The intervention is effective with no early graft losses or peri-operative vascular thromboses. The requirements for service provision are significant including the availability of additional transducers, and sonographers with expertise in intra-operative scanning able to attend after-hours for extended periods.
Introduction
The number of kidney transplants performed annually in Australia and New Zealand more than doubled from 540 cases in 2001 to 1104 in 2019.1 In a systematic review,2 patients undergoing intra-operative duplex scanning had improved early surgical outcomes with lower rates of early returns to theatre for graft ischaemia and reduced early graft loss.
In 2007, in our transplant unit, suddenly and unexpectedly, the urine output of a live donor kidney transplant recipient stopped. An urgent post-operative ultrasound was performed in the Recovery Ward but was suboptimal due to patient body habitus and swelling, and thus the underlying problem remained unclear. The patient was returned to the operating theatre, and real time ultrasound imaging using a sheathed hockey stick transducer placed directly on the kidneys and vessels, demonstrated stenosis at the arterial anastomosis, which was successfully revised with a satisfactory clinical outcome. On review, it was apparent that for this patient, intra-operative duplex scanning during the initial procedure would have identified the technical complication immediately and avoided a subsequent early return to theatre.
Our technique of intra-operative scanning has developed since, as familiarity with the technique, scan findings and clinical experience increased. Initially, only the arterial anastomosis and cortical perfusion were examined. Subsequently, the native iliac vessels and venous anastomoses were also examined, as well as the effect of repositioning the kidneys and of fascial closure on graft perfusion.
Intra-operative scanning has been performed in all renal grafts from 2013. In the 331 consecutive adult single grafts performed to October 2022,3 there have been no graft losses attributable to surgical or technical factors, comparing favourably to the 1% rate of graft loss recorded in Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) for concurrent procedures (data supplied on request by ANZDATA). There were also no documented venous or arterial thromboses in the first 7 days post-operatively, which also compares favourably with reported rates of 2–5% from overseas centres.4, 5
Although the intent of the scan has always been obvious, it is only relatively recently that the scanning protocol was formalised. We now present the protocol that has been in use since 2020, and the technical and logistical requirements needed to support this service. The purpose of this formalised protocol is to ensure that the entire kidneys and associated vasculature is assessed, with all relevant images stored and a formal worksheet completed. This protocol has three principal aspects: equipment, scan technique including worksheet and images recorded, and service delivery.
All patients examined gave informed consent and were participants in a Northern Sydney Local Health District Human Research Ethics Committee approved (2020-ETH00443) study assessing ultrasound in renal transplantation.
Equipment
A high-end ultrasound machine is required with colour and Pulsed Wave (PW) Doppler capability. In our unit, a Canon Aplio 800 ultrasound system with an i22LH8 hockey stick transducer and an i8CX1 matrix transducer is used. In our hospital, only fully sterilisable transducers are used intra-operatively because sheathed transducers reprocessed with high-grade disinfection are not compliant with the local Infection Control Policy. Cleaning and sterilisation of transducers is performed by the hospital Central Sterilisation Unit using Sterrad (Advanced Sterilisation products, Irvine CA, USA) as per the ultrasound manufacturer's guidelines. Transducers in a sterile sheath could be used if compliant with local infection control practices. Barcoded hockey stick and curved linear transducers are stored in separate sterile packs in a temperature and humidity compliant storage area in the ultrasound department. The sterile transducers are taken by the sonographer to the operating theatre at the time of surgery, opened by the scout nurse and passed into the sterile field.
Scan Technique and Images Recorded
The operating surgeon holds the transducer, placing it directly onto the kidneys or vessels (Fig. 1A) with the dedicated sonographer guiding the imaging process including image optimisation, measurements and acquisition of images relevant to the protocol (Table 1) and sonographer worksheet (Appendix S1).
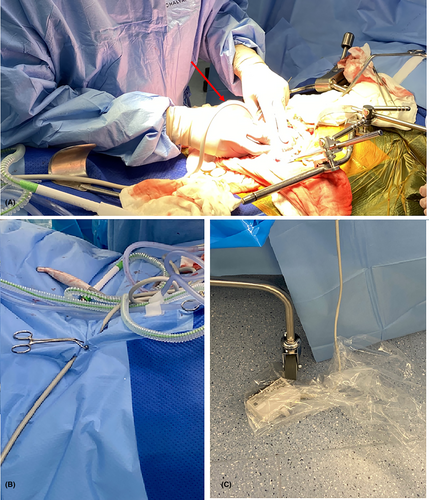
| Stage 1. Post-vascular anastomosis |
| Hockey stick transducer placed directly on the kidneys, then vessels |
|
|
|
|
| Stage 2. Post-ureteric anastomosis |
| Hockey stick transducer placed directly on the kidneys, then vessels as required |
|
Scanning is performed with the kidneys in the final position. Optimum perfusion is reconfirmed as the kidney position may have changed since the initial scan. If direct visualisation of major vessels requires moving the kidneys, rescan after the kidneys are replaced in position. |
| Stage 3. Closure scan |
| Hockey stick or curved transducers during, and after, fascial closure using transabdominal scanning technique scanning away from the incision with angulation towards the kidneys. Sterile gel may be required |
|
- Abbreviations: EIA, external iliac artery; EIV, external iliac vein; PSV, peak systolic velocity; PW, pulsed wave Doppler; RA, transplant renal artery; RI, cortical resistive index; RV, transplant renal vein; SMI, superb microvascular imaging.
There are three phases of scanning: Stage 1-post-vascular anastomosis, Stage 2-post-ureteric anastomosis and Stage 3-during and after fascial closure.
Stage 1: Post-vascular anastomosis.
This assessment occurs after the renal artery and vein clamps are released and aims to identify major vascular abnormalities to allow immediate surgical repair, or graft repositioning and avoid potential extended graft ischaemia. Fundamental ultrasound scanning principles are followed. The initial scan settings should be based on low velocity flow with colour and Pulsed Wave Doppler settings similar to those used for scanning small parts. Low velocity flow enhancing software such as superb microvascular imaging (SMI) (Canon Medical Systems, Tustin CA, USA) is of benefit but not essential. Prompt adjustments to scale settings between the cortical renal arteries with usual velocities of 3–15 cm/s and the transplant renal and native external iliac arteries with velocities of 100–400 cm/s are required.
The hockey-stick transducer is placed directly on the kidneys to examine perfusion and resistive indices of the cortex. No gel is required because there is sufficient fluid to act as an acoustic coupling medium, but if contact is unsatisfactory, warm irrigation fluid is used.
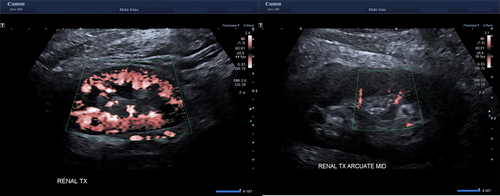
Renal cortical perfusion is qualitatively assessed by visually assessing the extent of colour (Fig. 2A) and SMI fill in the cortical vessels at the edge of the cortex. Pulsed wave Doppler (Fig. 2B) is used to quantitate resistive indices in the cortical arteries in the outer third of the cortex (Fig. 2C) in the upper, middle, and lower poles of the graft.
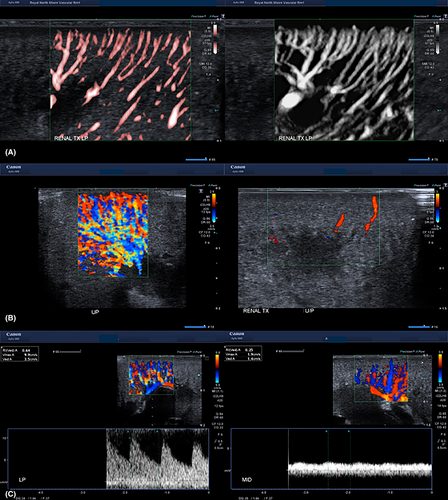
The transplant renal artery and vein are then assessed from the anastomosis to the renal hilum with B-mode, colour and pulsed wave Doppler. B-mode is used to image the vessel walls to identify plaque, fibromuscular dysplasia, dissection or thrombus. Colour Doppler is used to identify colour filling defects and sites of aliasing, and pulsed wave Doppler used to establish waveform profiles and velocities. The site and number of renal artery and vein measurements varies depending on surgeon preference and pathology, if any. When multiple renal arteries and veins are present, each vessel is insonated and images appropriately labelled. The anastomoses, and external iliac artery and vein, proximally and distally, are then assessed with a combination B-mode, colour Doppler and pulsed wave Doppler. The inferior epigastric artery, if used as in inflow vessel to perfuse an accessory renal artery, is also assessed. Arterial clamp sites, the most common location of dissection are specifically examined.
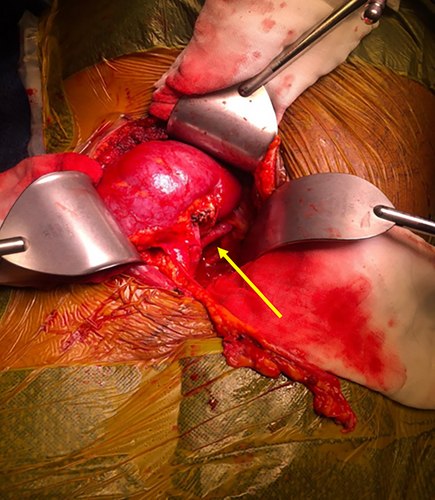
Whilst the full lengths of the major vessels are readily visualised with the kidneys retracted, these vessels are usually partially or completely obscured when the kidneys are placed in its final position in the pelvis (Fig. 3). Kinking or twisting of the vessels cannot be excluded despite the initial satisfactory perfusion. Cortical perfusion is therefore checked again with the graft in the proposed final position, providing an opportunity to reposition the graft with repeat scanning if perfusion is considered suboptimal.
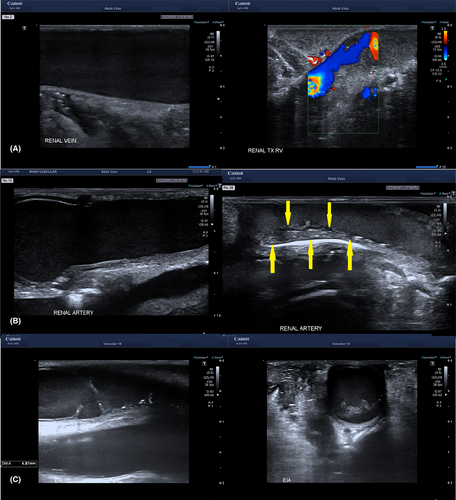
Expected normal findings are listed in Table 2. Abnormal ultrasound findings include renal vein thrombus or occlusion, renal artery stenosis or narrowing, vascular clamp injuries (Fig. 4A–C), as well as changes in perfusion following dynamic repositioning, closure-compression syndromes, and other conditions such as subcapsular haematoma, intra-renal cysts or tumours, air embolism, and anatomical variants (Table 2).
| Anatomic location | Expected normal | Abnormal ultrasound finding | If abnormal, consider |
|---|---|---|---|
| Renal cortex | Good perfusion to the edge of the cortex with cortical resistive indices (RIs) 0.50–0.70 | Tardis Parvus waveform | Severe arterial inflow restriction by stenosis or dissection in RA or EIA, or kinking or twisting of RA |
| High RI |
Venous outflow restriction by kinking or twisting of vein, compression by wound closure. Hyperacute rejection Poorer quality of graft setting baseline for post-operative scans |
||
| Homogeneous echotexture | Mobile echogenic intravascular foci | Air embolism from perfusion fluid | |
| Subcapsular haematoma | Compromised graft perfusion | ||
| Regional perfusion defect | Renal infarct | ||
| Irregular heterogenous focal lesion | Neoplasm | ||
| Renal artery |
Intact intima with no stenosis Low resistance waveform |
Triphasic waveform | Renal vein obstruction |
| RA/EIA velocity ratio <2 | High velocities or RA/EIA ratio >2 Luminal irregularity and narrowing | Fibromuscular dysplasia, dissection, or atheroma causing significant stenosis | |
| RA anastomosis | Not narrowed, intima regular | ||
| Renal vein | Phasic flow, no intraluminal thrombus | Loss of phasicity or no flow on PW, intraluminal thrombus | Anastomotic narrowing, vessel twisting or kinking with occlusion or pre-occlusion |
| RV anastomosis | Not narrowed | Narrowed with increased velocities | Anastomosis revision |
| External iliac artery | Intact intima with normal triphasic waveforms | Intimal irregularity on B-mode, high velocities on PW, narrow lumen | Dissection due to clamp injury |
| External iliac vein | Phasic flow with no intraluminal thrombus | Loss of phasicity | Proximal compression by retractors |
- Abbreviations: EIA, external iliac artery; EIV, external iliac vein; RA, transplant renal artery; RI, cortical resistive indices; RV, transplant renal vein.
The transducer remains in the operative field for the remainder of the case (Fig. 1B). In order to perform ureteric implantation, additional surgical access to the bladder and urinary catheter tubing is required, requiring the ultrasound machine to be moved. The transducer cable is therefore disconnected from the ultrasound machine, allowing the machine to be moved out of the way. There is also potential for leakage of urine or fluid from bladder irrigation, so the connector is placed under the operating table in a clean plastic bag (Fig. 1C).
Stage 2: Post-ureteric implantation.
After ureteric implantation, the hockey-stick transducer is reconnected to the ultrasound machine which is repositioned adjacent to the surgical field. The Stage 1 scan technique and image acquisition is repeated to confirm that the findings of the Stage 1 scan have been maintained, and that there has been no change in graft perfusion due to a change in graft position.
Stage 3: Closure scan.
The surgical team prepares for closure with placement of drains and analgesic nerve blocks, final checks of haemostasis and removal of retractors. During this stage a transabdominal scanning technique is used, with a sterilised curved linear transducer required and abdominal pre-set selected. Sterile gel is needed once the skin is closed. It is difficult to scan directly over the surgical incision due to edge shadowing so a scanning window away from the incision is often used. Scanning during and after fascial closure identifies changes in graft perfusion presumed to changes in graft position, vessel kinking or graft compression caused by the closure.
The kidneys are imaged and assessed in the longitudinal and transverse planes with B-mode to set a baseline for the size, shape and echogenicity of the graft. Colour Doppler is used to assess cortical perfusion and pulsed wave Doppler is used to assess cortical arterial waveform profiles and velocities. Renal artery and renal vein velocities and waveforms are obtained, if possible, according to surgeon preference and scanning skill. If there is a significant loss of colour perfusion to the edge of the cortex (Fig. 5), or the cortical artery RI has become abnormal, then complete or partial release of the fascial closure is performed until satisfactory perfusion has returned, with the defect closed by mesh.
The dedicated worksheet is completed and also records the imaging start and finish times of the various stages of scanning during the operation, the systolic and diastolic velocities, the cortical RI, major vessel flow parameters, the number and size of multiple renal arteries when present, additional anastomoses, anatomical variants and/or sites of absent or delayed cortical perfusion. The cortical perfusion and resistive indices are assessed at all stages, and other information according to surgeon preference. Any requirement for repositioning of the kidneys is defined by the surgical team. If two kidneys are implanted in a single recipient at the same procedure, separate worksheets and reports are completed for each kidney. Formal separate ultrasound reports are completed for each stage of the procedure: post-vascular anastomosis, post-ureter anastomosis and closing.
The success of this imaging technique relies on open discussion in real time6 between the transplant surgeon and the sonographer who must be trained and experienced in scanning renal transplants, understand both normal and abnormal Doppler waveforms, the mechanism of abnormal waveforms, and be experienced in guiding interventional procedures. Throughout the imaging and any subsequent inventions, the sonographer guides the surgeon and optimises machine applications. Thus, effective service provision requires the surgeon and sonographer to be collaborative, with active engagement of sonographers including availability when transplants are performed after-hours.
At Royal North Shore Hospital, Sydney, Australia, between January 2013 and December 2022, intra-operative ultrasound assessment identified otherwise unsuspected inflow abnormalities requiring immediate intervention in 7 of 331 grafts.3 Ultrasound confirmed suspected venous outflow restriction requiring revision in a further 4 cases, and guided graft repositioning in 32 cases, which on 4 occasions required anastomotic revision in order to position the graft with satisfactory perfusion.
Service Provision
Whilst some sonographers undertake ultrasound scanning in a hospital setting or participate in ultrasound guided interventions, few will have undertaken ultrasound scanning during open surgical procedures which require an understanding of the sterile operative field, and the roles of the many staff in the operating theatre during the procedure. The sonographer needs to be present soon after clamp release for the initial scan and remain available for serial scans during the procedure as required, and for the final scans at wound closure. The median (interquartile range) scan times of each of the three phases of the scans were 9 min (7–13), 4 min (2–9) and 5 min (2–12) with the overall time from the first to the last image 118 min (92–136).
Whilst live donor transplants are scheduled, and mostly performed within hours, scheduling for deceased donor kidney grafts is unpredictable due to the time and location of the organ retrieval, as well as the availability of operating theatres in the implanting hospital. In our hospital, the operative times of two-thirds of all transplants extends outside routine working hours. Our Vascular Ultrasound Department routinely operates over extended hours including scheduled scanning sessions on weekends with sonographers also rostered on-call to provide availability out-of-hours for transplants and emergency vascular ultrasounds.
The ultrasound machine remains in the operating theatre for the whole period of the operation rather than being returned to the Department. Our current practice is for the sonographer to remain in the operating suite until the last scan is completed rather than undertake work at other sites in the hospital. Additional transducers are required to maintain service delivery whilst the transducers used intra-operatively are being resterilised. Should there be an early return to theatre, the transducers used in the initial procedure will likely now be unsterile and another sterile transducer probably required.
Conclusions
Post-operatively, ultrasound remains the imaging modality of choice for renal transplants avoiding radiation and nephrotoxic contrast. Intra-operative ultrasound has the same benefits, and also allows for immediate surgical correction of identified abnormalities. Measurements and results of the intra-operative scan also establish baseline perfusion parameters for post-operative scans.
Although the investigation was first described in 1998,7 until now, there have been no published protocols, so replication and comparison of results is problematic. The purpose of this publication is to present our current locally developed formal protocol for intra-operative ultrasound assessments to assist other transplant units to undertake this investigation, which improves patient outcomes.3
Acknowledgement
Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




